Difference between revisions of "Curcumin"
(Added images) |
|||
| Line 55: | Line 55: | ||
==Preparation== | ==Preparation== | ||
| − | An alcoholic solution of curcumin can be extracted from the spice turmeric. | + | An alcoholic solution of curcumin can be extracted from the spice turmeric. There's no need to synthesize it from basic compounds. |
==Projects== | ==Projects== | ||
Revision as of 18:16, 5 April 2016
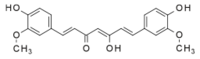
| |
| Names | |
|---|---|
| IUPAC name
(1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione
| |
| Properties | |
| C21H20O8 | |
| Molar mass | 400.385 |
| Appearance | Bright yellow-orange powder |
| Melting point | 183 °C (361 °F; 456 K) |
| Related compounds | |
| Related compounds
|
Rosocyanine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Curcumin is an organic compound found in the spice turmeric that can be used in the home lab as an acid-base indicator.
Contents
Properties
Chemical
Curcumin is a naturally occurring phenol with the chemical formula C21H20O6. This compound is useful as a pH indicator due to the normally yellow compound changing to red from pH 8.4 and higher. Hydrolysis of the molecule in very highly basic conditions may result in dark brown discoloration.
The reaction of curcumin with boric acid produces the ionic compound rosocyanine and the complex rubrocurcumin, both of which are deep red dyes. The former can be produced by dissolving curcumin and boric acid together in methanol and then adding a large amount of strong mineral acid, followed by briefly heating the mixture. A deep pure red solution is formed this way, and retains its color with dilution. Curcumin is a remarkably effective indicator for boron; sensitive to concentrations below 1ppm. Colorimetry can be used to make this a qualitative test.
Physical
Curcumin appears in its pure form as an intensely yellow-orange powder that is nearly insoluble in water but soluble in many alcohols. This makes it especially useful for the production of pH testing strips, as the curcumin in the strip will not leach out into an aqueous solution during testing.
Availability
Curcumin is very easily purchased in the form of turmeric, a common spice, and can be retrieved, along with other phenols, by extraction with alcohol.
Preparation
An alcoholic solution of curcumin can be extracted from the spice turmeric. There's no need to synthesize it from basic compounds.
Projects
- Make your own pH testing strips
- Synthesize the compound rosocyanine, which is a dark green solid that forms red solutions.
- Qualitatively determine the presence of borax in pool additives or laundry detergents.
Handling
Safety
Small doses as are found in food are considered safe. The nature of curcumin is not fully understood with respect to its role in the human body; large amounts may be carcinogenic. Apart from large, intentional doses, curcumin is safe to handle and utilize in the lab.
Storage
No special storage is required for curcumin.
Disposal
Curcumin can be safely poured down the drain.
