Difference between revisions of "2,4-Dinitrobromobenzene"
(Created page with "246x246px '''2,4 Dinitrobromobenzene''' is a useful organic intermediate and easier to prepare in a home lab than 2,4 dinitrochlorob...") |
|||
| Line 1: | Line 1: | ||
[[File:2,4-dinitrobromobenzene.png|thumb|246x246px]] | [[File:2,4-dinitrobromobenzene.png|thumb|246x246px]] | ||
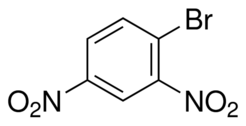
| − | '''2,4 Dinitrobromobenzene''' is a useful organic intermediate and | + | '''2,4-Dinitrobromobenzene''' is a useful organic intermediate and is readily prepared in a laboratory. |
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | 2,4 Dinitrobromobenzene is soluble in water and methanol. | + | 2,4-Dinitrobromobenzene is soluble in water and methanol. It is reactive towards reducing agents such as Sn/HCl and H<sub>2</sub>/Pd to form 2,4-diaminobromobenzene. |
===Physical=== | ===Physical=== | ||
| − | 2,4 | + | 2,4-Dinitrobromobenzene, when pure, is a light yellow crystalline substance which melts at 71°C-73°C. It has the characteristic slightly sweet smell of nitroaromatic compounds, though it is toxic and inhalation of the dust should be avoided. It has a density of 1.91 g/cm<sup>3</sup>. |
==Availability== | ==Availability== | ||
| − | 2,4 Dinitrobromobenzene is not offered by any suppliers available to the amateur. However, [[2,4 dinitrochlorobenzene]] may be available from chemical suppliers and | + | 2,4-Dinitrobromobenzene is not offered by any suppliers available to the amateur. However, [[2,4 dinitrochlorobenzene]] may be available from chemical suppliers and finds similar uses. |
==Preparation== | ==Preparation== | ||
| − | 2,4 | + | 2,4-Dinitrobromobenzene can be prepared with relative ease in a home lab by the nitration of [[bromobenzene]]. A writeup of this reaction is available [http://www.sciencemadness.org/talk/viewthread.php?tid=28480 here]. |
==Projects== | ==Projects== | ||
| − | *Preparation of [[2,4 Dinitrophenol]] as a precursor to [[DNPO]] | + | *Preparation of [[2,4-Dinitrophenol]] as a precursor to [[DNPO]] |
| − | *Preparation of [[2,4 Dinitrophenylhydrazine]] | + | *Preparation of [[2,4-Dinitrophenylhydrazine]] |
==Safety== | ==Safety== | ||
| − | 2,4 Dinitrobromobenzene is a mutagen and skin irritant. It may have shock sensitivity, though | + | 2,4-Dinitrobromobenzene is a mutagen and skin irritant. Skin contact should be avoided by wearing gloves as some people possess a severe allergy to the compound, resulting in contact dermatitis of varying severity. It may have shock sensitivity, though the extent of which is currently undocumented. |
==References== | ==References== | ||
Revision as of 00:45, 2 November 2015
2,4-Dinitrobromobenzene is a useful organic intermediate and is readily prepared in a laboratory.
Contents
Properties
Chemical
2,4-Dinitrobromobenzene is soluble in water and methanol. It is reactive towards reducing agents such as Sn/HCl and H2/Pd to form 2,4-diaminobromobenzene.
Physical
2,4-Dinitrobromobenzene, when pure, is a light yellow crystalline substance which melts at 71°C-73°C. It has the characteristic slightly sweet smell of nitroaromatic compounds, though it is toxic and inhalation of the dust should be avoided. It has a density of 1.91 g/cm3.
Availability
2,4-Dinitrobromobenzene is not offered by any suppliers available to the amateur. However, 2,4 dinitrochlorobenzene may be available from chemical suppliers and finds similar uses.
Preparation
2,4-Dinitrobromobenzene can be prepared with relative ease in a home lab by the nitration of bromobenzene. A writeup of this reaction is available here.
Projects
- Preparation of 2,4-Dinitrophenol as a precursor to DNPO
- Preparation of 2,4-Dinitrophenylhydrazine
Safety
2,4-Dinitrobromobenzene is a mutagen and skin irritant. Skin contact should be avoided by wearing gloves as some people possess a severe allergy to the compound, resulting in contact dermatitis of varying severity. It may have shock sensitivity, though the extent of which is currently undocumented.