Difference between revisions of "Acetaldehyde"
(→Preparation) |
|||
| Line 13: | Line 13: | ||
==Preparation== | ==Preparation== | ||
| − | Industrially, acetaldehyde is produced via the oxidation of [[ethylene|ethene]] over a copper-palladium catalyst. Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C: | + | Industrially, acetaldehyde is produced via the oxidation of [[ethylene|ethene]] over a copper-palladium catalyst. |
| + | |||
| + | :2 CH<sub>2</sub>=CH<sub>2</sub> + O<sub>2</sub> → 2 CH<sub>3</sub>CHO | ||
| + | |||
| + | Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C: | ||
:2 CH<sub>3</sub>CH<sub>2</sub>OH + O<sub>2</sub> → 2 CH<sub>3</sub>CHO + 2 H<sub>2</sub>O | :2 CH<sub>3</sub>CH<sub>2</sub>OH + O<sub>2</sub> → 2 CH<sub>3</sub>CHO + 2 H<sub>2</sub>O | ||
Revision as of 22:23, 6 December 2016
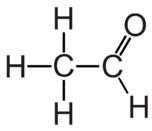
Acetaldehyde is the organic compound with chemical formula CH3CHO. It is the second-simplest aldehyde and finds use as a building block in organic synthesis.
Contents
Properties
Chemical
Depending on reaction conditions, the oxidation of acetaldehyde by oxygen variously co-produces acetic anhydride and acetic acid via the intermediate peracetic acid. The process relies on a catalyst containing metal ions and is typically conducted by introduction of gaseous oxygen into liquid acetaldehyde.
Physical
Acetaldehyde is a transparent, volatile, and extremely flammable liquid at room temperature that boils at only 20.2°C (68.4°F), making it difficult to store. The characteristic odor of acetaldehyde is sweet and reminiscent of green apple. Acetaldehyde has a flash point of only −39°C, making it absolutely crucial that any significant amount is kept away from possible ignition sources.
Availability
No over-the-counter source of acetaldehyde is known, and sources are unlikely due to the difficulty and danger of prolonged storage.
Preparation
Industrially, acetaldehyde is produced via the oxidation of ethene over a copper-palladium catalyst.
- 2 CH2=CH2 + O2 → 2 CH3CHO
Two routes exist from ethanol, the first an exothermic, self-sustaining oxidation reaction using a copper or silver catalyst at a temperature of 500-650°C:
- 2 CH3CH2OH + O2 → 2 CH3CHO + 2 H2O
The second method of preparing acetaldehyde from ethanol involves its dehydrogenation at a temperature of of 260-290 °C, again over a catalyst of copper. This route is endothermic, requiring constant and uniform heating, but has the advantage of not requiring oxygen input, which also reduces the risk of fire.
- CH3CH2OH → CH3CHO + H2[1]
Projects
- Pentaerythritol synthesis
Handling
Safety
Acetaldehyde is designated a probable carcinogen and is many times more toxic than ethanol, which it is a metabolite of. With a high chance of vaporization and a flash point of only −39 °C, acetaldehyde represents a significant fire hazard, and care should be made that all potential sources of ignition are kept away.
Storage
Acetaldehyde must be stored in a highly temperature-controlled environment given its low boiling point, and should also be kept in a separate cabinet or secondary container in a well-ventilated space, always away from potential sources of ignition.
Disposal
Acetaldehyde can be safely burned.
References
- ↑ Ullmann's Encyclopedia Of Industrial Chemistry, Wiley-VCH (2007)