Difference between revisions of "Fluorescein"
(→Synthesis) |
|||
| Line 137: | Line 137: | ||
===Synthesis=== | ===Synthesis=== | ||
| − | Fluorescein is easily prepared from [[resorcinol]] and [[phthalic anhydride]] through a [[Friedel-Crafts]] reaction with an acid catalyst, most often concentrated [[sulfuric acid | + | Fluorescein is easily prepared from [[resorcinol]] and [[phthalic anhydride]] through a [[Friedel-Crafts]] reaction with an acid catalyst, most often concentrated [[sulfuric acid]]. The product may be diluted into a basic solution to obtain a dark sodium fluorescein stock solution, which works for most demonstrations. |
==Projects== | ==Projects== | ||
Revision as of 19:25, 28 April 2018
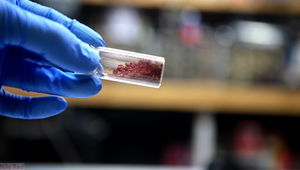 Freshly prepared fluorescein
| |
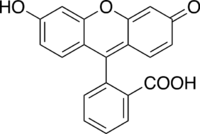 Structure of fluorescein
| |
| Names | |
|---|---|
| IUPAC name
3′,6′-dihydroxyspiro[isobenzofuran-1(3H),9′-[9H]xanthen]-3-one
| |
| Systematic IUPAC name
3',6'-Dihydroxy-3H-spiro[2-benzofuran-1,9'-xanthen]-3-one | |
| Other names
3,6-Fluorandiol
Fluorescein D&C yellow no. 7 Diresorcinolphthalein Japan yellow 201 Resorcinolphthalein Soap yellow Solvent Yellow 94 Yellow fluorescein | |
| Properties | |
| C20H12O5 | |
| Molar mass | 332.31 g/mol |
| Appearance | Dark red powder |
| Odor | Odorless |
| Density | 1.602 g/cm3 |
| Melting point | 314 to 316 °C (597 to 601 °F; 587 to 589 K) |
| Boiling point | Decomposes |
| 0.005 g/100 ml (20 °C) | |
| Solubility | Soluble in aq. alkali, acetone, diethyl ether, methanol, pyridine Slightly soluble in ethanol |
| Vapor pressure | 4.1·10-14 mmHg (25 °C) |
| Acidity (pKa) | 6.4 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fluorescein (also known as resorcinophthalein or D&C yellow no. 7) is an organic compound that is a brick-red solid at room temperature. It dissolves slightly in water to give bright yellow, strongly fluorescent solutions. Sodium fluorescein, also known as uranine, has a much higher solubility in water.
Despite its name, fluorescein is NOT a fluorine compound.
Contents
Properties
Chemical
Fluorescein will react with bicarbonates to form fluorescein salts.
Physical
Fluorescein is a dark red powder, slightly soluble in water. However, when dissolved in a very large amount of water, it will turn bright green, with the color getting stronger the more it dissolves (up to a point obviously), a property which gives it useful applications in various domains.
Fluorescence
Fluorescein has an absorption peak at 494 nm and an emission peak at 512 nm. Its isosbestic point (wavelength that is equally absorbed at all pH values) is 464 nm.
Availability
Fluorescein is sold by chemical suppliers. It can also be found on eBay and Amazon, at various prices.
One seller offers 100 g of fluorescein at $18.81.
Obtaining
Extraction
Certain brands of highlighters use a solution of fluorescein in water with a water-soluble binder. The ink cartridges of these highlighters may be extracted with isopropanol to give a solution of pure fluorescein. Some highlighters use pyranine instead, which has a strong blue fluorescence at high pH.
Bubble levels are often very dilute fluorescein solutions.
Synthesis
Fluorescein is easily prepared from resorcinol and phthalic anhydride through a Friedel-Crafts reaction with an acid catalyst, most often concentrated sulfuric acid. The product may be diluted into a basic solution to obtain a dark sodium fluorescein stock solution, which works for most demonstrations.
Projects
- Water flow indicator
- Demonstration of fluorescence
See also
Handling
Safety
Diluted fluorescein solutions are considered safe and are commonly used in eye examinations. Ingestion of concentrated solutions however can lead to nausea.
Storage
Solid fluorescein should be stored in closed bottles, away from moisture or corrosive vapors. Solutions should be kept in bottles, away from light.
Disposal
No special disposal is required.