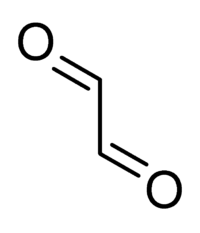
Glyoxal
| |
| Names | |
|---|---|
| IUPAC name
Ethanedial
| |
| Other names
Ethane-1,2-dial
Ethanedione Ketoethanal Oxalaldehyde Oxaldehyde | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C2H2O2 CHOCHO | |
| Molar mass | 58.036 g/mol |
| Appearance | Colorless to yellow solid, yellow liquid giving off greenish vapors (pure) Colorless liquid (solution) |
| Odor | Mild odor |
| Density | 1.14 g/cm3 (liquid) (20 °C)[1] 1.27 g/cm3 (40% aq. solution) (20 °C) |
| Melting point | 15 °C (59 °F; 288 K) [2] |
| Boiling point | 51 °C (124 °F; 324 K) |
| Miscible | |
| Solubility | Reacts with amines Miscible with alcohols, ethers, ketones |
| Vapor pressure | 18 mmHg (20 °C) |
| Hazards | |
| Safety data sheet | Sigma-Aldrich (40% aq. sol.) |
| Flash point | −4 °C (25 °F; 269 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
3,300 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Acetaldehyde Ethylene glycol Oxalic acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glyoxal is an organic compound with the chemical formula CHOCHO or C2H2O2. It is the simplest dialdehyde (a compound with two aldehyde groups).
Contents
Properties
Chemical
Pure glyoxal is said to burn in air/oxygen with a purple flame.
- C2H2O2 + 3/2 O2 → 2 CO2 + H2O
Pure glyoxal is not commonly encountered because it forms hydrates, which oligomerize. For many purposes, these hydrated oligomers behave equivalently to glyoxal.
Glyoxal is oxidized by dilute nitric acid to glyoxyl acid, while conc. nitric acid will oxidize it to oxalic acid.
Physical
Glyoxal is a volatile liquid that freezes just slightly below room temperature. The compound is interesting, because unlike other small weight CHO compounds, it is not colorless. The liquid and solid forms are yellow, while the vapors of this compound are green.
Availability
Glyoxal is sold as 40% solution by chemical suppliers.
Preparation
Glyoxal can be prepared by careful oxidation of ethanol with nitric acid.
Another lab route involves oxidation of acetaldehyde with selenous acid. Yield is given to be 70%. If selenium dioxide is used, the yield varies between 60-84%, and selenous acid/selenium dioxide is preferred over nitric acid, as it is more selective.[3]
Commercial glyoxal is prepared either by the gas-phase oxidation of ethylene glycol in the presence of a silver or copper catalyst (the Laporte process) or by the liquid-phase oxidation of acetaldehyde with nitric acid. Cooper oxide can be used as catalyst at 300 °C.[4]
Decarboxylation of dihydroxy-tartaric acid salts in the presence of sodium bisulfite has been shown to yield glyoxal.[5]
Ozonolysis of benzene has been shown to produce glyoxal.[6]
Anhydrous glyoxal is prepared by heating solid glyoxal hydrate(s) with phosphorus pentoxide and condensing the vapors in a cold trap.[7]
Another route to anhydrous glyoxal involves adding dichloroethane to oleum at 58-60 °C, in the presence of mercury(II) chloride as catalyst. Glyoxal sulfate is produced, which is neutralized with calcium carbonate to yield glyoxal.[8]
Projects
- Colored compound
- Preparation of imidazoles and other similar heterocyclic compounds
- Synthesis of HNIW
- Synthesis of glycoluril and tetranitroglycoluril (TNGU)
- Solubilizer and cross-linking agent in polymer chemistry
- Fixative for histology
- Wrinkle-resistant chemical treatments of clothing
Handling
Safety
Glyoxal is volatile, flammable, irritant and like most simple aldehydes, suspected carcinogen. Wear proper protection when working with this compound.
Storage
Should be kept at constant temperature. In solution may precipitate on storage, and can be redissolved by heating at 50-60 °C.
Disposal
Glyoxal can be neutralized by precipitating it with a base. Can also be mixed with acetone or ethanol and burned.
References
- ↑ Debus; Justus Liebigs Annalen der Chemie; vol. 102; (1857); p. 28
- ↑ Debus; Justus Liebigs Annalen der Chemie; vol. 102; (1857); p. 28
- ↑ http://www.orgsyn.org/demo.aspx?prep=cv3p0438
- ↑ http://www.inchem.org/documents/cicads/cicads/cicad57.htm#4.1
- ↑ https://books.google.ro/books?id=AQ42AQAAMAAJ&dq=editions:y5IMLeQql-4C&source=gbs_navlinks_s&redir_esc=y&hl=ro
- ↑ https://pubs.acs.org/doi/abs/10.1021/i360029a017
- ↑ https://zenodo.org/record/1426217#.XzrghFBS_IU
- ↑ Brudz, V. G.; Drapkina, D. A.; Markovich, I. S., Sb. Statei, Vses. Nauchn.-Issled. Inst. Khim. Reaktivov i Osobo Chistykh Khim. Veshchestv (1961), (No. 24), 98-101
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Aldehydes
- Volatile chemicals
- Carcinogenic
- Contact poisons
- Liquids
- Air-sensitive materials