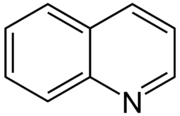
Quinoline
| |
| Names | |
|---|---|
| IUPAC name
Quinoline
| |
| Systematic IUPAC name
1-Benzopyridine | |
| Other names
2-Azabicyclo[4.4.0]deca-1,3,5,7,9-pentaene
1-Azanaphthalene 1-Benzazine 1-Benzine Benzazabenzene Benzazine Benzo[b]azine Benzopyridine Chinoleine Chinoline Leucoline Leukol | |
| Properties | |
| C9H7N | |
| Molar mass | 129.16 g/mol |
| Appearance | Colorless to yellowish oily liquid |
| Odor | Penetrating, fish-like |
| Density | 1.093 g/cm3 (20 °C) |
| Melting point | −15 °C (5 °F; 258 K) |
| Boiling point | 237.7 °C (459.9 °F; 510.8 K) |
| 0.61 g/100 ml (20 °C) | |
| Solubility | Miscible with acetone, benzene, carbon disulfide, diethyl ether, ethanol |
| Vapor pressure | 0.06 mm Hg at 25 °C |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
174.9 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | 101 °C (214 °F; 374 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
262 mg/kg (rat, oral) |
| Related compounds | |
| Related compounds
|
Pyridine |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N.
Contents
Properties
Chemical
Oxidation of quinoline with potassium permanganate yields quinolinic acid (pyridine-2, 3-dicarboxylic acid).
The reduction of quinoline with sodium borohydride in the presence of acetic acid gives Kairoline A.
Physical
Quinoline is a colorless liquid, that darkens upon contact with air. It is not miscible with water, but more miscible with organic solvents. Its odor is similar to most amines, though not as unpleasant as pyridine.
Availability
Quinoline is sold by chemical suppliers.
Preparation
Quinoline can be prepared from aniline and glycerol via the Skraup reaction.
Projects
- Preparation of 8-Hydroxyquinoline
- Preparation of quinolinic acid
- Preparation of quinine
Handling
Safety
Quinoline is irritant and should be handled with care.
Storage
In closed bottles, away from light and air.
Disposal
Can be neutralized with Fenton's reagent.
Can also be destroyed by incineration in a special oven.