Difference between revisions of "Sodium hydroxide"
(→Chemical) |
|||
| Line 196: | Line 196: | ||
[[Category:Irritants]] | [[Category:Irritants]] | ||
[[Category:Air-sensitive materials]] | [[Category:Air-sensitive materials]] | ||
| + | [[Category:Materials unstable in acidic solution]] | ||
Revision as of 18:28, 31 March 2019
 Sodium hydroxide beads
| |
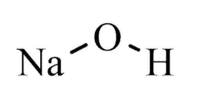
| |
| Names | |
|---|---|
| IUPAC name
Sodium hydroxide
| |
| Other names
Ascarite
Caustic soda Lye Sodium hydrate White caustic | |
| Properties | |
| NaOH | |
| Molar mass | 39.9971 g/mol |
| Appearance | White oily solid |
| Odor | Odorless |
| Density | 2.13 g/cm3 |
| Melting point | 318 °C (604 °F; 591 K) |
| Boiling point | 1,388 °C (2,530 °F; 1,661 K) |
| 41.8 g/100 ml (0 °C) 111 g/100 ml (20 °C) 337 g/100 ml (100 °C) | |
| Solubility | Soluble in ethanol, glycerol, methanol Almost insoluble in ammonia Insoluble in diethyl ether |
| Solubility in ethanol | 13.9 g/100 ml |
| Solubility in methanol | 23.8 g/100 ml |
| Vapor pressure | <2.4 kPa (at 20 °C) |
| Thermochemistry | |
| Std molar
entropy (S |
64 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−427 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
40 mg/kg (mouse, intraperitoneal) |
| Related compounds | |
| Related compounds
|
Lithium hydroxide Potassium hydroxide Rubidium hydroxide Caesium hydroxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium hydroxide, sometimes called caustic soda, is a strongly basic inorganic compound with chemical formula NaOH. It is a hygroscopic white solid with a variety of uses, such as in double replacement reactions to form metal hydroxides or oxides.
Contents
Properties
Physical
Sodium hydroxide is an odorless hygroscopic white solid. Due to its hygroscopic nature, sodium hydroxide will readily absorb enough water from the air to dissolve itself; for this reason, samples are hard to weigh accurately in dry form and standardized solutions are often used for more precise chemistry. The dissolution of sodium hydroxide into water is very highly exothermic; lower-quality glassware can even break from thermal stress if this reaction proceeds too quickly. Sodium hydroxide is soluble in water, methanol, ethanol, glycerol, but insoluble in most ethers. Alcoholic solutions of sodium hydroxide will oxidize in air, turning brown.
Chemical
Concentrated solutions of sodium hydroxide also have the unique property of being one of the few things that can dissolve glass, and even dilute solutions will do this over long time. Molten sodium hydroxide is much more powerful and will dissolve glass much faster, forming sodium silicate.
- 2 NaOH + SiO2 → Na2SiO3 + H2O
For this reason, sodium hydroxide should be stored only in thick plastic containers.
Sodium hydroxide is also unique among basic compounds in that it reacts vigorously with amphoteric metals, including aluminium and some transition metals, producing large quantities of hydrogen gas and heat as it reacts.
- 2 NaOH + 2 Al → 2 NaAlO2 + H2
Sodium hydroxide does not attack magnesium or other base metals.
Sodium hypochlorite, which is not available in appreciable concentrations in many countries, can be prepared by channeling chlorine gas into aqueous sodium hydroxide, forming a solution strong and pure enough to produce chloroform or potassium chlorate.
- 2 NaOH + Cl2 → 2 NaClO + H2O
In a process known as saponification, which is used in soapmaking, sodium hydroxide can hydrolyze organic esters such as those found in naturally occurring plant or animal fats, producing fatty acid salts and glycerol.
- 3 NaOH + ((CH2)nCOO)3CH2CHCH2 → 3 (CH2)nCOONa + OH-CH2CH(OH)-CH2OH
Sodium hydroxide tends to absorb acidic vapors and/or carbon dioxide from the air to form the salt of the acid absorbed or in case of carbon dioxide, sodium carbonate if left out for a while, particularly in solution. If stored with another acid, it will form a sodium salt. While sodium bicarbonate is usually seen as a base, if present with sodium hydroxide in solution it will act as an acid, neutralizing it to produce sodium carbonate as well.
- 2 NaOH + CO2 → Na2CO3 + H2O
If the air is polluted, calcium sulfite/sulfate and calcium nitrate may also be produced.
Sodium hydroxide and sometimes other strong bases are used to scrub gases such as hydrogen chloride/fluoride/bromide, hydrogen sulfide, nitrogen dioxide (but not nitric oxide), or sulfur oxides. This is done by passing those gases into a solution of sodium hydroxide, effectively neutralizing them and allowing some syntheses to be performed indoors and outside of a fume hood with a proper setup. Multiple scrubbers may be required when working with large amounts of harmful gases.
Molten sodium hydroxide is extremely corrosive. It tends to react with almost all metals, except a few like copper, nickel, and alloys like stainless steel, though presence of air and moisture may increase the rate of corrosion. Magnesium oxide and thorium dioxide are the only few metallic oxides that are not affected by molten alkali. Molten sodium hydroxide will oxidize platinum metal and reacts with gold forming a sodium-gold alloy.
Molten sodium hydroxide is one of the few reagents that rapidly dissolve glass, forming sodium silicate. It will also dissolve many other metal oxides, resulting in metal oxide anions, such as silicates, ferrates, aluminates.
Preparation and Acquisition
Sodium hydroxide can be produced via the chloralkali process, an electrolysis setup in which sodium chloride solution is electrolyzed in the cathode half of a membrane cell, generating chlorine and hydrogen gases, while Na+ ions are generated in solution that pass through the membrane, where they are reduced to form sodium hydroxide. Because chlorine is also produced in this process, the product is likely to be contaminated with varying amounts of sodium hypochlorite.
Alternatively, sodium hydroxide can often be bought in the form of drain cleaners at hardware stores; however, any other agents are often difficult to remove from drain cleaners that aren't 100% caustic lye, as they are often intended to react with the sodium hydroxide during the drain-cleaning process. Many formulas, however, have different reagents coming in particles of different size, i.e. sodium hydroxide as large-ish pellets or flakes, and aluminium as fine powder; such mixtures are easy to separate using sieves. Other types of caustic soda tend to have ~10% sodium chloride. The cheapest available sodium hydroxide for purchase is typically found online, however, from suppliers such as Duda Diesel ($3/lb). Some hardware stores will sell very clean sodium hydroxide pellets or flakes, usually cheap. Brand products that contain sodium hydroxide flakes however tend to be more expensive weight per weight than bulk caustic soda bought from most hardware stores.
Due to the typical high cost of sodium hydroxide from hardware stores in many places, it may be a better idea to synthesize it from calcium hydroxide and sodium carbonate, if such materials are available. This method requires producing a saturated solution of calcium hydroxide and adding a stoichiometric amount of sodium carbonate, precipitating calcium carbonate in the process.
- Na2CO3 + Ca(OH)2 → CaCO3 + 2 NaOH
Once boiled or evaporated to near dryness, neither of which should be done in glassware due to the destructive nature of sodium hydroxide solutions, the sodium hydroxide can be purified by decanting the concentrated solution into ethanol or methanol, precipitating out any leftover calcium hydroxide. If very pure sodium hydroxide is needed, it is recommended that slightly less than the required amount of sodium carbonate is used in the initial reaction.
Uses
One of the most common applications of sodium hydroxide in introductory chemistry is as a strong base for acid-base titrations; if a standard solution is prepared from a known quantity of sodium hydroxide, titration can be used to determine the concentration of an acid solution. Sodium hydroxide is also a convenient base for producing other sodium salts from acids, as it does not evolve any gases upon neutralization. The production of many metal oxides and hydroxides can be accomplished by mixing solutions of sodium hydroxide and soluble metal salts. Finally, sodium metal can be prepared from sodium hydroxide in two ways. In the first, hot molten sodium hydroxide is electrolyzed, depositing sodium on the electrodes. In the second, a mixture of fine, dry sodium hydroxide is mixed with magnesium powder and ignited in a thermite-like reaction. The most common use of sodium hydroxide is in soap making industry.
Handling
Safety
Sodium hydroxide, like other strong bases, is highly corrosive to skin and flesh. As such it should be carefully handled and safety procedures should be planned in advance. Neutralization with a weak acid and persistent washing with hot water should be carried out until the skin no longer feels slippery.
The melting point of sodium hydroxide (318°C) is not that high, so any open flame and some hot plates are able to melt the solid. Molten sodium hydroxide is extremely dangerous. It will dissolve glass, violently react with aluminium, corrode steel and eat through flesh with ease. A splattering of molten hydroxide is capable of causing horrific wounds, so appropriate safety measures, such as a face shield and lab coat, must be taken when dealing with the molten material.
Storage
Sodium hydroxide pellets or flakes should be stored in thick plastic container or stainless steel if possible, and sealed, to prevent them from absorbing water, carbon dioxide and other gasses from the surrounding air. NEVER store sodium hydroxide in aluminium containers, as it react with it, nor ordinary steel, as it will rust, and while not all grades will get completely corroded, it will contaminate the hydroxide.
Sodium hydroxide solutions should only be stored in glass bottles for a certain period of time, as it will absorb carbon dioxide or sulfur gasses from air and will eventually neutralize itself. They will also slowly attack glass. Sodium hydroxide solutions will slowly creep sodium carbonate outside the vessel if kept in contact with air, and open containers can be completely covered after several months. Even closed bottles will form some sodium carbonate, as it is very difficult to make the bottle air-tight. As such, aqueous NaOH solutions should not be kept for more than a few months.
Disposal
Sodium hydroxide can be neutralized with any acid, though using a weak acid, such as vinegar or citric acid is more economic. Sodium bicarbonate can also be used. As long as they're not contaminated with hazardous impurities, sodium hydroxide solutions can be poured down the drain, as most cleaning products contain NaOH.