Difference between revisions of "Sulfur trioxide"
(Added SMILES code.) |
|||
| (19 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Sulfur trioxide''', chemical formula SO<sub>3</sub>, is a highly corrosive and easily melted white solid at room temperature. It is the acid anhydride of sulfuric acid. | + | {{Chembox |
| + | | Name = Sulfur trioxide | ||
| + | | Reference = | ||
| + | | IUPACName = Sulfur trioxide | ||
| + | | PIN = | ||
| + | | SystematicName = Sulfonylideneoxidane | ||
| + | | OtherNames = Sulfur(VI) oxide<br>Sulfuric anhydride | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Solid sulfur trioxide in ampoule by ChemicalForce.jpg | ||
| + | | ImageSize = 300 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = A large glass ampoule containing crystallized SO<sub>3</sub> | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = O=S(=O)=O | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White crystalline solid or liquid, which fumes in air | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 45 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = 1.96 g/cm<sup>3</sup> (10 °C)<br>1.929 g/cm<sup>3</sup> (15 °C)<br>1.911 g/cm<sup>3</sup> (20 °C)<br>1.893 g/cm<sup>3</sup> (25 °C)<br>1.875 g/cm<sup>3</sup> (30 °C)<br>1.857 g/cm<sup>3</sup> (35 °C)<br>1.84 g/cm<sup>3</sup> (40 °C)<br>1.809 g/cm<sup>3</sup> (45 °C)<br>1.783 g/cm<sup>3</sup> (50 °C)<br>1.7552 g/cm<sup>3</sup> (55 °C) | ||
| + | | Formula = SO<sub>3</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 80.066 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 16.9 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | Odor = Sulfurous, highly corrosive | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = Reacts highly exothermically | ||
| + | | SolubleOther = Reacts with [[alcohol]]s, [[ester]]s<br>Soluble in bromine trifluoride, [[bromoacetic acid]], [[dichloromethane]], halocarbons, [[hydrogen cyanide]], cold [[nitromethane]]<ref>Appel, R.; Goehring, M.; Zeitschrift fuer Anorganische und Allgemeine Chemie; vol. 271; (1953); p. 171 - 175</ref>, sulfuryl chloride fluoride, [[tetrachloroethylene]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = 263 mmHg at 25 °C | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = -371.17 kJ/mol | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = −395.7 kJ/mol | ||
| + | | Entropy = 256.77 J·K<sup>-1</sup>·mol<sup>-1</sup> | ||
| + | | HeatCapacity = 210 J·K<sup>-1</sup>·mol<sup>-1</sup> | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = Non-flammable | ||
| + | | ExploLimits = Non-explosive | ||
| + | | ExternalMSDS = [https://www.docdroid.net/Nhgey0o/sulfur-trioxide-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = Non-flammable | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = Highly corrosive | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Sulfur dioxide]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Sulfur trioxide''', chemical formula '''SO<sub>3</sub>''', is a highly corrosive and easily melted white solid at room temperature. It is the acid anhydride of [[sulfuric acid]]. | ||
==Properties== | ==Properties== | ||
===Chemical=== | ===Chemical=== | ||
| − | Sulfur trioxide is an extremely strong dehydrating agent that causes immediate and highly exothermic charring of virtually any organic material it comes into contact with. Sulfur trioxide reacts with water in the air to form a dense fog of concentrated sulfuric acid. | + | Sulfur trioxide is an extremely strong dehydrating agent that causes immediate and highly exothermic charring of virtually any organic material it comes into contact with. Sulfur trioxide reacts with water in the air to form a high;y dangerous and corrosive dense fog of concentrated sulfuric acid. |
Sulfur trioxide also reacts with [[sulfur dichloride]] to yield [[thionyl chloride]]. | Sulfur trioxide also reacts with [[sulfur dichloride]] to yield [[thionyl chloride]]. | ||
| Line 9: | Line 119: | ||
===Physical=== | ===Physical=== | ||
| − | Sulfur trioxide is a volatile liquid that fumes in contact with open air. It has a relative narrow liquid range, melting at 16.9 °C and boiling at 45 °C. | + | Sulfur trioxide is a volatile liquid that fumes in contact with open air. This compounds reacts violently with water and alcohols, releasing a fine mist of sulfuric acid and alkyl sulfates. It has a relative narrow liquid range, melting at 16.9 °C and boiling at 45 °C, meaning that under standard conditions it may be encountered as liquid or as a solid.<ref>https://www.youtube.com/watch?v=grV8IvIqeUs</ref> |
==Availability== | ==Availability== | ||
| Line 15: | Line 125: | ||
==Preparation== | ==Preparation== | ||
| − | + | The preparation of this compound is extremely dangerous and should only be attempted by chemists with experience working with hazardous volatile reagents. | |
| − | + | SO<sub>3</sub> can be made in low yield through the pyrolysis of [[sodium persulfate]], first forming [[ozone]] and then SOSO<sub>3</sub>. A catalytic amount of 100% sulfuric acid is required. | |
| − | In industry sulfur trioxide is produced via the [[contact process]]. Purified sulfur dioxide is mixed with dry air and injected through a bed of [[Vanadium | + | Nearly the same reaction takes place when sodium bisulfate is very strongly heated, first evolving water, forming [[sodium pyrosulfate]], which then decomposes above 460 °C to sodium sulfate, releasing SO<sub>3</sub>. |
| + | |||
| + | There are reports that heating sodium pyrosulfate with concentrated sulfuric acid to 150°C results in SO<sub>3</sub> and bisulfate. | ||
| + | |||
| + | Heating iron(II) sulfate at 700 °C with carbon yields iron(III) oxide, sulfur dioxide and sulfur trioxide. The same reaction also works with [[iron(III) sulfate]], at a much lower temperature, 480 °C, and produces mostly sulfur trioxide. | ||
| + | |||
| + | Pyrolysis of [[copper(II) sulfate]] above 560 °C yields sulfur trioxide and copper oxide. If the temperature gets lower, copper sulfate will reform. [[Aluminium sulfate]] also works, though the decomposition temperature is slightly higher. | ||
| + | |||
| + | Adding [[phosphorus pentoxide]] to extremely concentrated sulfuric acid will release sulfur trioxide, which can be extracted via distillation. Metaphosphoric acid can also be used instead of the pentoxide. | ||
| + | |||
| + | Roasting [[calcium sulfate]] with [[silicon dioxide]] (very fine sand can be used) at 1000 °C for 1 hour yields calcium silicate and sulfur trioxide. Adding small amounts of [[chromium(III) oxide]] or tungsten(IV) oxide improves the process.<ref>Adadurov, L. E.; Pligunov, V. P.; Zhurnal prikladnoi Khim. (russ.); vol. 5; (1932); p. 149 - 156</ref> | ||
| + | |||
| + | In industry sulfur trioxide is produced via the [[contact process]]. Purified sulfur dioxide is mixed with dry air and injected through a bed of [[Vanadium pentoxide|V<sub>2</sub>O<sub>5</sub>]] catalyst heated to temperatures between 400-600 °C (the optimum temperature is 450 °C), at 1-2 atm. The resulting hot sulfur trioxide is recirculated and passed through more layers of catalyst to increase the yield. The gaseous SO<sub>3</sub> is cooled by passing it through a heat exchanger and can be collected if required. The condensed trioxide is dissolved in concentrated H<sub>2</sub>SO<sub>4</sub> in the absorption tower to form oleum. | ||
| + | |||
| + | Sulfur trioxide can also be obtained by oxidizing sulfur dioxide in the presence of several metal oxides, such as [[copper(II) oxide]] at high temperatures<ref>http://pubs.rsc.org/en/content/articlelanding/1917/ct/ct9171100379#!divAbstract</ref> or chromium(III) oxide (at temperatures between 180-400 °C).<ref>http://onlinelibrary.wiley.com/doi/10.1002/jlac.18480660118/abstract</ref> | ||
==Projects== | ==Projects== | ||
| Line 38: | Line 162: | ||
Due to its relative narrow liquid range, between 16.9 °C 45 °C, it's mandatory to keep sulfur trioxide away from any source of heat, although if kept in a place too cold it will freeze. | Due to its relative narrow liquid range, between 16.9 °C 45 °C, it's mandatory to keep sulfur trioxide away from any source of heat, although if kept in a place too cold it will freeze. | ||
| + | |||
| + | Sulfur trioxide has a tendency to violently polymerize, much faster in contact with moisture, and samples should never stored without inhibitors. Some inhibitors used are [[antimony pentafluoride]] or [[antimony pentachloride]].<ref>https://www.google.com/patents/US2511072</ref> | ||
===Disposal=== | ===Disposal=== | ||
| − | SO<sub>3</sub> | + | The neutralization of SO<sub>3</sub> is, to put it bluntly, a nightmare. Simply exposing it to air will cause copious amounts of sulfuric acid fumes to be produced, which are highly dangerous and corrosive, meaning that just pouring the substance from its bottle/container is an extremely hazardous choice. In order to safely transfer it from a container to another, you will have to either employ a PTFE cannula, or an acid-resistant syringe. It reacts explosively with water and most bases, so dilution must be performed very slowly. The sulfate salt can then be washed down the sink. A safer way involves slowly adding sulfur trioxide over dry calcium carbonate powder, which results in anhydrous calcium sulphate and carbon dioxide. However this process produces large amounts of heat, which causes some sulfur trioxide to become airborne, forming a dangerous corrosive mist. Neutralizing SO<sub>3</sub> is not something that can be done in lab, and instead it should be done in either a special enclosed chamber fitted with washing shower or outside in the presence of draft. Neutralizing large amounts of sulfur trioxide is very dangerous for the amateur chemist. |
| + | |||
| + | A relatively safe method of SO<sub>3</sub> neutralization is dissolving it in copious amounts of concentrated sulfuric acid. This is best done in two parts: first, you dissolve it in 98% sulfuric acid, resulting in [[oleum]]. Then you neutralize oleum by adding it to 70-80% sulfuric acid, resulting in more 98-100% sulfuric acid. Both processes are very exothermic and corrosive fumes of sulfuric acid will still be produced to a certain degree, so it's best to do this in a safe location, wearing proper lab protection. The dilution route is the industrial method concentrated sulfuric acid and oleum are made in chemical plants. | ||
==References== | ==References== | ||
| Line 59: | Line 187: | ||
[[Category:Corrosive chemicals]] | [[Category:Corrosive chemicals]] | ||
[[Category:Choking agents]] | [[Category:Choking agents]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 23:45, 25 August 2020
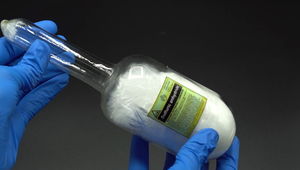 A large glass ampoule containing crystallized SO3
| |
| Names | |
|---|---|
| IUPAC name
Sulfur trioxide
| |
| Systematic IUPAC name
Sulfonylideneoxidane | |
| Other names
Sulfur(VI) oxide
Sulfuric anhydride | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| SO3 | |
| Molar mass | 80.066 g/mol |
| Appearance | White crystalline solid or liquid, which fumes in air |
| Odor | Sulfurous, highly corrosive |
| Density | 1.96 g/cm3 (10 °C) 1.929 g/cm3 (15 °C) 1.911 g/cm3 (20 °C) 1.893 g/cm3 (25 °C) 1.875 g/cm3 (30 °C) 1.857 g/cm3 (35 °C) 1.84 g/cm3 (40 °C) 1.809 g/cm3 (45 °C) 1.783 g/cm3 (50 °C) 1.7552 g/cm3 (55 °C) |
| Melting point | 16.9 °C (62.4 °F; 290.0 K) |
| Boiling point | 45 °C (113 °F; 318 K) |
| Reacts highly exothermically | |
| Solubility | Reacts with alcohols, esters Soluble in bromine trifluoride, bromoacetic acid, dichloromethane, halocarbons, hydrogen cyanide, cold nitromethane[1], sulfuryl chloride fluoride, tetrachloroethylene |
| Vapor pressure | 263 mmHg at 25 °C |
| Thermochemistry | |
| Std molar
entropy (S |
256.77 J·K-1·mol-1 |
| Std enthalpy of
formation (ΔfH |
−395.7 kJ/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Flash point | Non-flammable |
| Related compounds | |
| Related compounds
|
Sulfur dioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfur trioxide, chemical formula SO3, is a highly corrosive and easily melted white solid at room temperature. It is the acid anhydride of sulfuric acid.
Contents
Properties
Chemical
Sulfur trioxide is an extremely strong dehydrating agent that causes immediate and highly exothermic charring of virtually any organic material it comes into contact with. Sulfur trioxide reacts with water in the air to form a high;y dangerous and corrosive dense fog of concentrated sulfuric acid.
Sulfur trioxide also reacts with sulfur dichloride to yield thionyl chloride.
- SO3 + SCl2 → SOCl2 + SO2
Physical
Sulfur trioxide is a volatile liquid that fumes in contact with open air. This compounds reacts violently with water and alcohols, releasing a fine mist of sulfuric acid and alkyl sulfates. It has a relative narrow liquid range, melting at 16.9 °C and boiling at 45 °C, meaning that under standard conditions it may be encountered as liquid or as a solid.[2]
Availability
SO3 is available for purchase only to professional chemists due to its extreme hazards. It is commonly sold as a solution in sulfuric acid known as oleum.
Preparation
The preparation of this compound is extremely dangerous and should only be attempted by chemists with experience working with hazardous volatile reagents.
SO3 can be made in low yield through the pyrolysis of sodium persulfate, first forming ozone and then SOSO3. A catalytic amount of 100% sulfuric acid is required.
Nearly the same reaction takes place when sodium bisulfate is very strongly heated, first evolving water, forming sodium pyrosulfate, which then decomposes above 460 °C to sodium sulfate, releasing SO3.
There are reports that heating sodium pyrosulfate with concentrated sulfuric acid to 150°C results in SO3 and bisulfate.
Heating iron(II) sulfate at 700 °C with carbon yields iron(III) oxide, sulfur dioxide and sulfur trioxide. The same reaction also works with iron(III) sulfate, at a much lower temperature, 480 °C, and produces mostly sulfur trioxide.
Pyrolysis of copper(II) sulfate above 560 °C yields sulfur trioxide and copper oxide. If the temperature gets lower, copper sulfate will reform. Aluminium sulfate also works, though the decomposition temperature is slightly higher.
Adding phosphorus pentoxide to extremely concentrated sulfuric acid will release sulfur trioxide, which can be extracted via distillation. Metaphosphoric acid can also be used instead of the pentoxide.
Roasting calcium sulfate with silicon dioxide (very fine sand can be used) at 1000 °C for 1 hour yields calcium silicate and sulfur trioxide. Adding small amounts of chromium(III) oxide or tungsten(IV) oxide improves the process.[3]
In industry sulfur trioxide is produced via the contact process. Purified sulfur dioxide is mixed with dry air and injected through a bed of V2O5 catalyst heated to temperatures between 400-600 °C (the optimum temperature is 450 °C), at 1-2 atm. The resulting hot sulfur trioxide is recirculated and passed through more layers of catalyst to increase the yield. The gaseous SO3 is cooled by passing it through a heat exchanger and can be collected if required. The condensed trioxide is dissolved in concentrated H2SO4 in the absorption tower to form oleum.
Sulfur trioxide can also be obtained by oxidizing sulfur dioxide in the presence of several metal oxides, such as copper(II) oxide at high temperatures[4] or chromium(III) oxide (at temperatures between 180-400 °C).[5]
Projects
- SO3 reacts with SCl2 to form thionyl chloride
- SO3 reacts (violently) with water to form sulfuric acid, with dilute sulfuric acid to form concentrated sulfuric acid, and with the concentrated acid to form oleum.
- A mixture of SO3 and sulfuric acid is effective at sulfonating aromatic compounds, forming useful reagents like benzenedisulfonic acid and toluensulfonic acid.
Handling
Safety
Sulfur trioxide is highly corrosive and will fume in open air, resulting in a highly dangerous and corrosive sulfuric acid mist. Since this mist is incredibly hazardous, standard protection clothing is insufficient, as the acid mist will attack most organic fibers as well as wreck most air filtration systems and may condense on the cloth. A showering installation may be required in the worst case scenario.
Sulfur trioxide reacts violently with plain water 1.
Storage
Sulfur trioxide is best stored as a solution in sulfuric acid, also known as disulfuric acid.
Pure sulfur trioxide should only be stored in sealed ampoules, as it will corrode most metal and plastic containers and their lids. Teflon containers, sealed with PTFE tape can also be used, but are very expensive. Lastly, SO3 containers can also be kept in a glovebox, to limit the hazard in the event of spill.
Due to its relative narrow liquid range, between 16.9 °C 45 °C, it's mandatory to keep sulfur trioxide away from any source of heat, although if kept in a place too cold it will freeze.
Sulfur trioxide has a tendency to violently polymerize, much faster in contact with moisture, and samples should never stored without inhibitors. Some inhibitors used are antimony pentafluoride or antimony pentachloride.[6]
Disposal
The neutralization of SO3 is, to put it bluntly, a nightmare. Simply exposing it to air will cause copious amounts of sulfuric acid fumes to be produced, which are highly dangerous and corrosive, meaning that just pouring the substance from its bottle/container is an extremely hazardous choice. In order to safely transfer it from a container to another, you will have to either employ a PTFE cannula, or an acid-resistant syringe. It reacts explosively with water and most bases, so dilution must be performed very slowly. The sulfate salt can then be washed down the sink. A safer way involves slowly adding sulfur trioxide over dry calcium carbonate powder, which results in anhydrous calcium sulphate and carbon dioxide. However this process produces large amounts of heat, which causes some sulfur trioxide to become airborne, forming a dangerous corrosive mist. Neutralizing SO3 is not something that can be done in lab, and instead it should be done in either a special enclosed chamber fitted with washing shower or outside in the presence of draft. Neutralizing large amounts of sulfur trioxide is very dangerous for the amateur chemist.
A relatively safe method of SO3 neutralization is dissolving it in copious amounts of concentrated sulfuric acid. This is best done in two parts: first, you dissolve it in 98% sulfuric acid, resulting in oleum. Then you neutralize oleum by adding it to 70-80% sulfuric acid, resulting in more 98-100% sulfuric acid. Both processes are very exothermic and corrosive fumes of sulfuric acid will still be produced to a certain degree, so it's best to do this in a safe location, wearing proper lab protection. The dilution route is the industrial method concentrated sulfuric acid and oleum are made in chemical plants.
References
- ↑ Appel, R.; Goehring, M.; Zeitschrift fuer Anorganische und Allgemeine Chemie; vol. 271; (1953); p. 171 - 175
- ↑ https://www.youtube.com/watch?v=grV8IvIqeUs
- ↑ Adadurov, L. E.; Pligunov, V. P.; Zhurnal prikladnoi Khim. (russ.); vol. 5; (1932); p. 149 - 156
- ↑ http://pubs.rsc.org/en/content/articlelanding/1917/ct/ct9171100379#!divAbstract
- ↑ http://onlinelibrary.wiley.com/doi/10.1002/jlac.18480660118/abstract
- ↑ https://www.google.com/patents/US2511072
Sciencemadness Library
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Inorganic compounds
- Sulfur compounds
- Oxides
- Inorganic acid anhydrides
- Materials that react with water
- Materials unstable in basic solution
- Corrosive chemicals
- Choking agents
- Liquids