Difference between revisions of "Tetrachlorocuprate"
From Sciencemadness Wiki
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
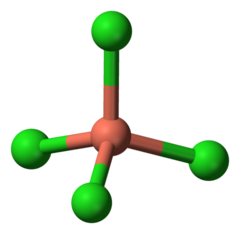
| − | [[File:Tetrachlorocuprate.png|thumb|240px|Tetrachlorocuprate structure]] | + | [[File:Tetrachlorocuprate structure.png|thumb|240px|Tetrachlorocuprate structure]] |
The tetrachlorocuprate ion is a [[Chloro complex|chloro complex]] of copper, represented by the formula '''[CuCl<sub>4</sub>]<sup>2-</sup>'''. | The tetrachlorocuprate ion is a [[Chloro complex|chloro complex]] of copper, represented by the formula '''[CuCl<sub>4</sub>]<sup>2-</sup>'''. | ||
Revision as of 19:00, 16 April 2017
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
The tetrachlorocuprate ion is a chloro complex of copper, represented by the formula [CuCl4]2-.
Properties
The tetrachlorocuprate ion is hard to get in solid form, but is rather easy to calcinate. Tetrachlorocuprate ions react violently with aluminium to form fine copper and aluminium(III) ions. In solution, the tetrachlorocuprate ion is bright green.
Production
This complex is generally produced by the addition of copper (II) ions to chloride ions. One easy method is by ading copper metal to a solution of hydrochloric acid and hydrogen peroxide, which forms tetrachlorocupric acid, the acid of the tetrachlorocuprate ion.
Projects
- PCB etchant
- Make coordination complexes