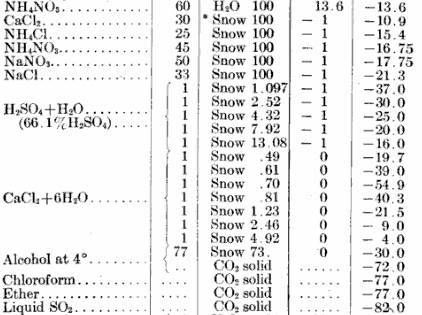
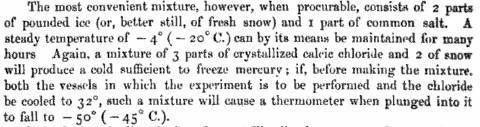I wonder if you could use different solvents, and different salts to condense liquid air? Use water/salt to freeze a gas like H2S, then use the frozen
H2S (or something else) to freeze another gas, mix it with a salt, and so forth to very, very low temps?
I know the number of steps involved would be large, and some device to hold all the mixtures, each with reducing volume, would be hard (if not
impossible to build) but imagine the rewards!
Liquid O2 made with reusable solvents, salts, and a freezer! |