
solo - 15-8-2008 at 18:32
I have been recently working on both the 2-benzylaziridines and the arylaziridines in the removal of the OH group from both the benzyl type alcohol on
amino alcohols and the primary OH on primary alcohol. Both of the methods depend on making a sulfuric ester by treatment of the base with conc. H2SO4
prefferaly cold acid, so the reaction doesn't get too hot and evaporate the reactant. I tried the process with both ephedrine and phenylalaninol
....both amino alcohols and had sorry results.....I don't know if it was the failed easy production of the ester or a very slow reduction of both the
arylaziridine and the 2-benzylaziridine catalytic hydrogenation procedure with no way to speed up the hydrogenation.There are several studies on the
reduction that have been posted on the reference section .....maybe I'll dig them up and post them to stimulate the dialogue on this thread........so
has anyone have any success stories with this process ...........solo
-----------------------------------------------------------------------------------
Synthesis of Arylaziridinesl
Stanley J. Brois
J. Org. Chem. 1962; 27(10); 3532-3534.
http://mihd.net/phb06o9

Abstract
Contrary to several reports in the chemical literature, the Wenker method has been successfully employed in the synthesis
of 1- and 2-arylaziridines. Thus, four typical 1-phenyl-2-amino-1-alkanolsa nd 2-anilinoethanol were readily esterified
with sulfuric acid in quantitative yields. Cyclization of the intermediary sulfate esters with alkali gave the desired phenylsubstituted
ethylenimines which were assayed for purity by gas chromatography and characterized by n.m.r. and infrared
spectroscopy. The n.m.r. data for 2-phenylaziridine (styrenimine) and styrene oxide strongly suggest that geminal couplings
in three-membered heterocycles depend not only on angular factors but also on the heteroatom.
--------------------------------------------------------------
2-Benzylaziridines. Cyclic Analogs of Amphetamines
Keith Brewster and Roger M. Pinder
Journal of Medicinal Chemistry1972, Vol. 15, No. 10, pg.1078
http://ifile.it/trwxukn
[2-benzylaziridine)

Excerp
Aziridines exhibit a wide spectrum of biological properties
and have found clinical application as antineoplastic
agents.' In addition to interaction with cell constituents,
the ability of aziridines to act as alkylating agents is reflected
in drug-receptor interactions; for example, 2-haloalkylamines
like dibenamine undergo cyclization in vivo to,
aziridinium ions prior to alkylation of the catecholamine
related compounds3-' prompted a study of the effects upon
their biological activity of incorporation of part of the
aminopropane side chain into an aziridine ring. 2-Benzylaziridine
(Ia) has been described6 but pharmacological data
are not available. We now report the synthesis and pharmacology
of this compound and its 4-chloro (Ib) and 4-
methoxy (IC) derivatives.
Note: there exists a thread on the subject at.....
http://wetdreams.ws/forum/topic.asp?TOPIC_ID=2946&Search...
[Edited on 16-8-2008 by solo]
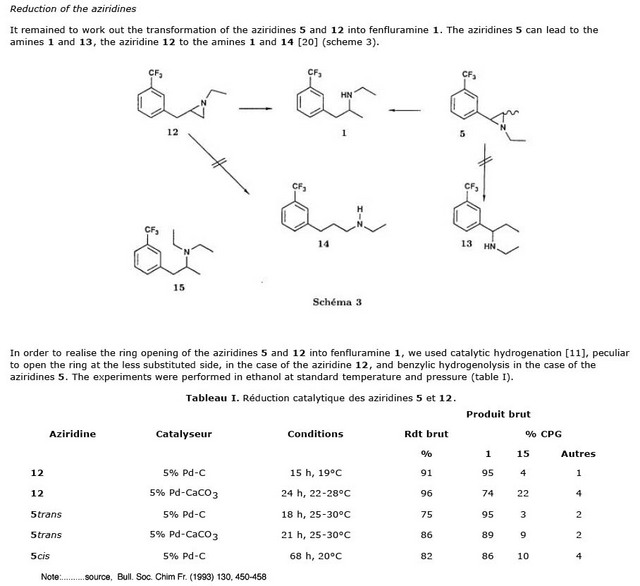
solo - 30-8-2008 at 15:22
As the hydrogenation of this material is in question ....I wonder if the purification of the imine to remove potential poisons for the Pd as commented
in ................
https://sciencemadness.org/talk/viewthread.php?tid=9872
can be the reason for the failure to hydrogenate the material.....some used raney nickle to tie up the sulfur might just do the trick.....solo
Klute - 30-8-2008 at 18:21
Maybe you could use something more efficient than H2SO4 to form a leaving group, such as TsCl? This would mean that the cyclization would happen
quicker/more easily. But if the the low step is the reduction od the aziridine, it might not be such an improvement and more a waste of TsCl. A
micro-scale trail would help you sort that out (reduction or cylization causing problems).
Is there nay other reducting agent capable of working? You could also prepare a small sample via LiAlH4 or soemthing, and use TLC to see if the
reaction just never starts (possibly unsuitable catalyst), or starts off well and then comes to a halt (poisonning of the catalyst). I'm sure a
treatment with activated carbon or raney nickel can only help?
Good luck with this one!


