


Quote: Originally posted by NurdRage  |
Quote: Originally posted by bfesser  |

 Pure enough for me.
Pure enough for me.



 By the way, those are the
actual colors-even though the copper looks dull.
By the way, those are the
actual colors-even though the copper looks dull.Quote: Originally posted by Megamarko94  |
Quote: Originally posted by blogfast25  |


Quote: Originally posted by Megamarko94  |
Quote: Originally posted by LanthanumK  |
Quote: Originally posted by MrHomeScientist  |


Quote: Originally posted by condennnsa  |
Quote: Originally posted by condennnsa  |
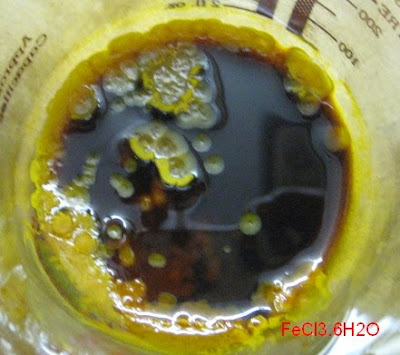
 <sub>2</sub>.6H<sub>2</sub>O may well be the compound you
stumbled upon. It would be nice to have analytical certainty though. Determining the amount of crystal water would be one element of analysis that
could confirm (or infirm) the hypothesis that your stuff is indeed CuK<sub>2</sub>(SO<sub>4</sub>
<sub>2</sub>.6H<sub>2</sub>O may well be the compound you
stumbled upon. It would be nice to have analytical certainty though. Determining the amount of crystal water would be one element of analysis that
could confirm (or infirm) the hypothesis that your stuff is indeed CuK<sub>2</sub>(SO<sub>4</sub> <sub>2</sub>.6H<sub>2</sub>O. For this, weight accurately some
of the dry hydrate crystals, then drive off the water by heating and weigh again. From the weight loss we could then determine if the hydrate water at
least corresponds to the alleged formula.
<sub>2</sub>.6H<sub>2</sub>O. For this, weight accurately some
of the dry hydrate crystals, then drive off the water by heating and weigh again. From the weight loss we could then determine if the hydrate water at
least corresponds to the alleged formula.


 , made 5 grams of pure white needles of p-bromoacetanilide and am currently making bromobenzene by rxn of KBrO3 in dil. H2SO4 with benzene.
, made 5 grams of pure white needles of p-bromoacetanilide and am currently making bromobenzene by rxn of KBrO3 in dil. H2SO4 with benzene.Quote: Originally posted by Squall181  |
Quote: Originally posted by barley81  |
Quote: Originally posted by Xenomorph  |

Quote: Originally posted by Jor  |

Quote: Originally posted by Ozone  |

Quote: Originally posted by blogfast25  |
 .
.
Quote: Originally posted by strontiumred  |
| Quote: |




 One was traded to Theo Gray.
One was traded to Theo Gray. 


Quote: Originally posted by Endimion17  |
Quote: Originally posted by m1tanker78  |


Quote: Originally posted by bob800  |
Quote: Originally posted by Wizzard  |
Quote: Originally posted by watson.fawkes  |
 Somebody with a large autoclave did, and these were
produced as the process was being learned, judging by the shapes.
Somebody with a large autoclave did, and these were
produced as the process was being learned, judging by the shapes.Quote: Originally posted by Wizzard  |

Quote: Originally posted by blogfast25  |
Quote: Originally posted by sternman318  |
