
calamus - 5-12-2010 at 16:16
where i could obtain a reffrance TLC plate
anyone have any idea
i wanna compare unknown sample with known one
Ozone - 5-12-2010 at 18:02
So far as I know, there is no such thing.
You would take a TLC plate and add, as one (or more) of the spots, a known (reference) compound. This compound is usually either a precursor or a
bona-fide sample of the product you intend to isolate/synthesize. Once developed, the retention factor (RF) of the compound would be compared agaist
the unknown, usually spotted onto the same plate. Frequently, this method is used to quickly monitor a synthesis or to assay column fractions for
product(s).
Here's some introductory (this should go into Beginnings) information on thin layer chromatography:
http://orgchem.colorado.edu/hndbksupport/TLC/TLC.html
I attached a drawing which I hope will help.
Cheers,
O3
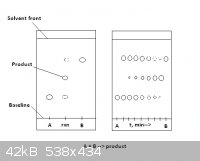
crazyboy - 5-12-2010 at 19:01
Unfortunately TLC is no good for identifying unknown compounds. It can tell you if they are polar or non polar but it won't identify them unless you
suspect it is a compound of which you have an authentic sample. The Rf value will change depending on the solvent system used so you can't use Rf
alone as an identifier. Furthermore many compounds have Rf values that are close or very similar to each other.
To identify your unknown properties such as melting point, boiling point, density and solubility are all good places to start, just make sure your
unknown compound is pure and not a mixture of several compounds.
[Edited on 6-12-2010 by crazyboy]
aonomus - 5-12-2010 at 20:08
Typically when you run a TLC plate, you want to run some reference standards against it on the same plate. The solvent system used to run your TLC
plates can vary from run to run, and its even dependent on the temperature of the system, and the moisture content of the silica on the plates.
The most common use of TLC is to monitor reaction completeness. You can take a TLC plate and spot it with your starting materials, and spot it with
your reaction mixture as you continue to heat it. By this method you can monitor the disappearance of starting materials, and the appearance of
products and byproducts.
calamus - 24-12-2010 at 09:46
thanks Ozone ,crazyboy &aonomus
thanks for your guidance
