
Arcaeca - 10-9-2019 at 21:26
So having never taken a biochemistry or molecular biology course, I decided it would be a cool idea to try to make some carminic acid from scratch.
(Because I want carmine to use as red pigment, but I live nowhere near any species of cochineal, and just buying carmine isn't fun).
According to this paper:
https://www.sciencedirect.com/science/article/pii/S096517481...
Carminic acid can (theoretically, at least) be synthesized starting from flavokermesic acid (FKA in the paper), hydroxylating to yield kermesic acid,
and then glycosylating to yield carminic acid, and then I gather I would need to precipitate the carmine pigment out with an aluminum salt.
And flavokermesic acid, AKA laccaic acid D, apparently, can be found in rhubarb rhizomes and "green vegetables" (although, unhelpfully, it doesn't say which green vegetables? Spinach? Broccoli?)
So first, I have to find a vegetable that contains flavokermesic acid. I'll do some more digging. Or, I could literally dig, as in a garden, and plant
some rhubarb, but... meh.
Second, I would somehow need to extract the flavokermesic acid. I couldn't find much solubility data on it, but this patent for laccaic acid extraction (granted, from lac resin, not vegetables, by the looks of it) says "laccaic acid itself has only a
solubility in water 0.03g (20 ° C) or so", and from its structure I suspect it would be moderately soluble in organic solvents like acetone, so
reflux the vegetables in an acetone bath?
This is where I get lost. The first step is hydroxylating one of the aromatic rings on the anthraquinone base of flavokermesic acid, but I was
flipping through my old O-chem notes and couldn't find anything about arene hydroxylation; closest thing would be OH- causing nucleophilic
substitution with a better leaving group like Cl, but then I'd have to chlorinate the ring, and if I remember right that would involve amination with
NH3 > diazotation with nitric acid > N2 falls off leaving an aromatic carbocation > add HCl and the Cl- swoops in and attaches.
Although... I guess why even bother with the Cl-, and just attach OH- directly by adding NaOH?
Is there no less convoluted way to do this, especially since I have no idea how the rest of the flavokermesic acid's functional groups would react
with the NH3 and nitric acid?
And that still leaves the glycosylation. I have an aqueous glucose solution (made by decomposing sucrose with heat and citric acid), but I have no
idea how to attach it. This is where my o-chem knowledge runs out.
Doable or fool's errand?
Tsjerk - 10-9-2019 at 23:43
I've only checked the 1991 synthesis, so I don't know whether different paths have been discovered in the mean time. So doable, but not easy.
DOI:10.1039/C39910001319
Use sci-hub.tw
Arcaeca - 11-9-2019 at 20:22
Yeah, but if I remember right they start with anthraquinone, and I'm not trying to do a "total" synthesis - I just want carminic acid from the
easiest-to-acquire ingredients as possible. And starting with flavokermesic acid seems to cut down on the number of steps significantly.
OH- is a stronger nucleophile than H, but I suppose it would be naive to think that simply adding NaOH to a flavokermesic acid solution
would cause the ring to undergo nucleophilic substitution? (To be clear, I'm referring to the flavokermesic -> kermesic acid part of the sequence
in the attachement; kermesic acid has one more OH group)
What if I did a process similar to benzene > phenol where you first add sulfuric acid to sulfonate the ring to get benzenesulfonic acid, then add
enough NaOH to neutralize it to sodium benzenesulfonate, and then add excess NaOH to get sodium phenolate, and finally acid to reprotonate the
phenolate to get phenol? (Of course, flavokermesic acid instead of benzene) I guess one problem is flavokermesic acid has 3 open spots for the
sulfuric acid to attack and I have no guarantee that it would attack the right place to make kermesic acid, so it would likely produce a large amount
of waste.
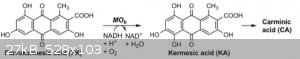
Tsjerk - 11-9-2019 at 23:11
Hydroxide groups are meta/para directors, as all phenols have at least a meta position available I guess you will get a mixture of all three isomers.
Boffis - 12-9-2019 at 00:01
Mmmm thats a pretty complex synthesis. The main problem I see is getting that disilyl substituted pentadiene compound and the pyrane derivative won't
be easy to prepare either.
I think it may be easier to extract it from the cochineal insects, you can buy dried and sterilized ones on line. I bought some from a place in the US
that supplies home textile dyers with natural colours and pigments. I may even still have the name.
Yep. the company is called Aurora Silk, they are online.
[Edited on 12-9-2019 by Boffis]
