
walruslover69 - 13-12-2019 at 06:08
I have been working in a lab using a few different Alkoxide salts. I've noticed that when bought (98%) most of them are either yellowish orange, or if
prepared (Sodium metal and alcohol) they turn yellow/orange fairly quickly when exposed to air.
This isn't a huge problem. but I am very curious as to the actual reaction that is taking place and the products of it.
What is the reaction of, for example Sodium Methoxide with air or water in the air that causes the color change. I'm aware of the simple hydrolysis to
NaOH and methanol. Is it some type of polymerization or potentially forming peroxide?
Side not: I believe the yellow impurity to also be fluorescent. A solution in DMSO glows a blueish green under 365nm UV light. The fluorescent peak is
at ~460nm and the UV-Vis absorbance peak is at ~375nm I can upload some PL and UV-Vis spectra if that would help.
[Edited on 13-12-2019 by walruslover69]
AJKOER - 13-12-2019 at 14:12
Actually, I would suspect it is the action of light, likely lab light rich in UV, as CH3OH is reputedly a good source of radicals:
CH3OH + hv = .CH3 + .OH
This reference https://pubs.acs.org/doi/pdf/10.1021/ja00865a053 claims the present of alkoxide radical created on photolysis, so with methanol, the .CH3O radical
and the metal in an excited state. The latter acting on O2 may create a peroxide as you suggested.
So clearly, in the presence of UV light and O2, products and decomposition reactions expected.
[Edited on 13-12-2019 by AJKOER]
Metacelsus - 13-12-2019 at 19:42
The reference AJOKER posted isn't relevant.
I think the most likely explanation for the color is air oxidation to aldehydes, followed by aldol condensation to various unsaturated compounds. But
for methoxide it would be more complicated since formaldehyde would be the initial product. I would like to see the spectra if you have them.
G-Coupled - 13-12-2019 at 21:09
I'd also like to see the spectra, if you have them available - this is really interesting. 
walruslover69 - 14-12-2019 at 11:19
Here is the absorbance and Fluorescence of Sodium Ethoxide. The PL was taken at 330,360,380,400,420,480 and 510nm.
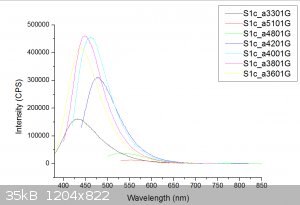
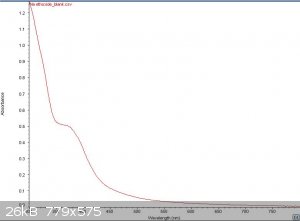
DavidJR - 14-12-2019 at 11:56
I've never noticed a yellow colour when preparing sodium alkoxides (I've made NaOMe, NaOEt, NaOiPr). I also have some commercial solid NaOMe from
sigma which has not turned yellow even with air exposure.
walruslover69 - 14-12-2019 at 14:04
I didn't have any problems making NaOEt from sodium and ethanol, but I found making Sodium hexanoxide (sodium+1 Hexanol) it was much more sensitive .
It was white while the sodium was reacting but as soon as the sodium was consumed the warm solution started to turn yellow and as a deep orange withen
an hour. I later did the reaction on a schlenk line and vacuum distilled the remaining hexanol off and it worked. But doing a regular distillation
resulted in an orange product.
Pumukli - 14-12-2019 at 14:22
Several months ago I mixed NaOH with MeOH in a flask and closed it. Did the same with i-propanol. The i-propanol - NaOH mixture turned orangish yellow
in 3 months. The MeOH containing mixture is still white.

