
clearly_not_atara - 7-2-2020 at 11:17
https://www.thieme-connect.com/products/ejournals/pdf/10.105...
The Mukaiyama reagent is already one of the easiest coupling agents to synthesize: 2-chloropyridine reacts with methyl iodide. That's it! Direct
reaction of pyridine with Cl2 even proceeds at 2 (in the gas phase at 270 C). However, pyridine is already annoying to make, and Cl2 is kind of scary,
and the simplicity of this moiety begs for innovation.
The reductive cleavage of thiamin was brought to my attention originally by someone interested in the thiazole fraction, but the pyrimidine can also
be recovered. Unfortunately the usual method with bisulfite leaves a sulfonic acid moiety attached, which hampers solubility in aprotic solvents.
However, the cleavage can also be effected by other, less problematic nucleophiles, as described in the attached paper by Zoltewicz et al.
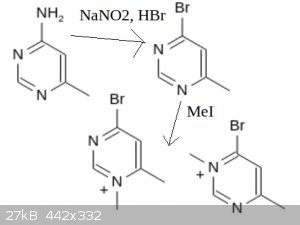
If a suitably inert pyrimidine is obtained, it is only two steps away from the active bromopyrimidinium. This can then be used to couple a variety of
carboxylic acids with a variety of nucleophiles. The picture I drew isn't quite substituted right, but you get the idea.
Some reports even mention that ketenes can be obtained by decomposition of the pyri[mi]dinium ester intermediate! This provides an unusually
facile route to versatile intermediates like phenylketene.
Attachment: zoltewicz1984.pdf (822kB)
This file has been downloaded 340 times
[Edited on 7-2-2020 by clearly_not_atara]
monolithic - 9-2-2020 at 05:55
Can you dumb it down for me and show some examples of what this reagent can do? 
clearly_not_atara - 9-2-2020 at 08:44
The link at the top of the post shows several examples with refs. A particularly dramatic example is the conversion of beta,gamma-unsaturated acids to
cyclobutanones:
https://pennstate.pure.elsevier.com/en/publications/generati...
I should mention that a similar starting material can be obtained by the reaction of guanidine and acetylacetone to 2-amino-4,6-dimethylpyrimidine,
which can be diazotized and methylated to a similar halopyrimidinium reagent.
Boffis - 22-2-2020 at 11:21
Hi c_n_atara, I've been looking into the degradation of thiamine and the resulting products. Rather fascinationg but it looks like the amino group may
not function in quite the normal fashion and may not yield a diazonium compound but merely undergo hydrolysis. I may be wwrong but in one of the
papers I found (and there are lots of them!) it reports that the pyrimidine part reacts with nitrous acid to give only nitrogen.
Could another nucleophile be used instead of sulphite? The paper you posted above gives another example of sodium azide to give the appropriate azide.
Maybe this could be reduced to an amine or eliminated by other means.
clearly_not_atara - 22-2-2020 at 13:54
It's likely that the diazonium is very unstable. As such I'm not surprised that nitrous acid gives a pyrimidone in many cases. Also, the sulfite gives
a sulfonic acid, so if you work with that it might have extra side rxns. Nitrosyl bromide might be an option if nothing else works.
For other nucleophiles I don't know. I would try methoxide first.
AvBaeyer - 22-2-2020 at 19:33
clearly_not_atara:
I know that if you dig a bit deeper you will find that the chemistry of pyridines (the basis of the Mukaiyama reagents) differs considerably from
pyrimidines. As Boffis infers above, there is a vast literature on pyrimdine chemistry, in particular aminopyrimidines and their reactions. Without
further information your post is merely speculative. Why not do the chemistry proposed and report back to us? Real chemistry, real results - how
novel.
AvB

