
nitro-genes - 1-12-2020 at 11:52
There seem to be lots of threads dealing with the synthesis of chlorates and perchlorates by electrolysis, though no topic exists about
electrosynthesis of nitrates from urea or ammonia.
Most articles concerning electrolytic ammonia oxidation seem to be aimed at nitrogen removal from urine as N2(g) and describe low yields (11%) of
nitrates (based on ammonia) using a simple graphite/graphite based anode/cathode cell (https://doi.org/10.1016/j.watres.2014.11.031). Overall, it seems difficult to avoid conversion to N2(g) perhaps due to some sensitive
hydroxylamine intermediate (soluble transition metals?) or lower barrier for cathodic reduction of nitrates as compared to chlorates. Relative high
nitrate production rates and yields seem feasible though: https://doi.org/10.1039/C7EW00014F
Another review on electrolytic ammonia oxidation mentions: "In neutral solutions (also at high anodic potentials), urea was decomposed mainly to
nitrite and nitrate ions and resulted in CO2 generation" Referrencing to a Russian article that I could not find online:
Osetrova, N. V., and A. M. Skundin. "Anodic oxidation of urea in neutral solutions." Russian journal of electrochemistry 30.10 (1994):
1145-1147.
Would anyone be able to download this one and add it to SMDB?  Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water.
Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water.
Any thoughts whether this would be efficiently feasible and if yes, what would be the best conditions for high nitrate conversion? Overall, a near
neutral pH seems best for nitrate synthesis, but overall it seems only small concentrations of nitrate can be realized before cathodic reduction
becomes significant. From the articles referenced above it also seems an interesting question what the role of chloride is in ammonia oxidation. Also
read something about lead/silver or nickel based anodes being especially advantageous, refs will follow. Would be cool if we could get this to work,
especially seen the importance of nitrates/nitration in amateur chemistry. Preventing cathodic reduction somehow or precipitating the nitrates (as a
quaternary ammonium salt maybe?) could help.
Instead of urea, an ammonium (bi)carbonate solution would also seem convenient (might be more conductive as solution)...blow CO2 through ammonia until
pH is only slightly alkaline --> perform electrolysis using a porous graphite anode and small surface graphite cathode at 10-30 mA/cm2 -->
distill off ammonium carbonate for recycling...any nitrate should be left as ammonium nitrate...Doable? 
[Edited on 1-12-2020 by nitro-genes]
mysteriusbhoice - 1-12-2020 at 20:59
You need sodium perchlorate catalyst for this process and also membrane cell and PbO2 electrode membrane for preventing nitrate reduction at cathode
and PbO2 is the only electrode capable of surviving nitric acid and ozone with perchlorate
nitro-genes - 2-12-2020 at 06:58
Hi mysteriusbhoice, why do you suggest PbO2 anode? Do you think high oxygen overpotential of the anode is important? Are you aware of any references
using PbO2 anodes for electrolysis of ammonia/urea/amines? Not sure it would survive or not be inactivated during electrolysis.  I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates.
I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates.
Here a couple of relevant articles it seems.
Article 2 seems interesting...again they mention that electrolysis of neutral sulfate or phosphate buffered solutions of urea using platinum
electrodes and high current densities results in selective conversion to nitrate and nitrite with almost 90% current efficiency. Also encouraging
seems that even with very small urea concentrations anodic oxygen evolution does not seem to be significant. Figure 2 seems to suggest that the
nitrate selectivity only applies to dilute solutions unfortunately, with increasing urea and/or nitrate concentrations oxidation to N2 becomes
increasingly more significant, pointing towards cathodic reduction or other detrimental reactions, such as polymerization of the urea (described
better in part 1: Osetrova, N. V., and A. M. Skundin. "Anodic oxidation of urea in neutral solutions." Russian journal of electrochemistry 30.10
(1994): 1145-1147.)
Article 3 looked at the effect of temperature and NaF vs NaCl additions on the efficiency of nitrate conversions. Overall, the presence of chloride in
these setups seemed to increase oxidation to N2(g) relative to nitrate, meaning direct conversion of urine would probably be less efficient.
Interestingly, NaF seems very efficient over a large urea concentration range, though nothing is stated about effect of total amp.h delivered, pH of
electrolyte or nitrate concentrations involved. I would expect aqueous NaF to behave relatively inert during electrolysis (similar to sulfates), but
maybe there is some interaction with the platinum electrodes?
Still have to let it sink in somewhat, though nitrate synthesis by electrolysis seems possible at least! 
Attachment: Anodic oxidation urea russian 2.pdf (39kB)
This file has been downloaded 354 times
Attachment: Anodic oxidation urea russian 3.pdf (24kB)
This file has been downloaded 341 times
[Edited on 2-12-2020 by nitro-genes]
mysteriusbhoice - 6-12-2020 at 10:33
som1 told me to try using cyanuric acid instead of urea for anodic oxidation
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
this thread talks about using melamine and cyanuric acid is that without the amines but still has the amides.
maybe mix of cyanuric acid and ammonia salts??
https://pubs.acs.org/doi/10.1021/es102423u#:~:text=The%20rea...
there is also that thread which talks about the chlorination of urea using Cl2 gas and I have tried chlorate electrolyte instead of NaCl and it
produced an acidic solution which eats copper but its incredibly dillute.
Now I think PbO2 and perchlorate electrolyte might help as a good catalyst since perchlorate electrolyte can emit ozone and perhaps help aid the
oxidation of urea hopefully not into N2.
I wanted to continue doing with chlorate however I notice a precipitate form that seemed to be unstable when collected it was NH4ClO3 and I was like
NOOOPE.
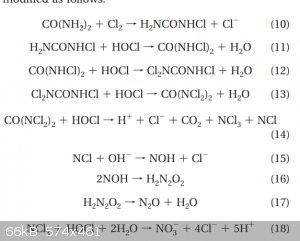
The chlorination of urea produces NCl3 and at high temps it hydrolyzes into NO2 and that makes HNO3.
Ive also snapped a screenshot of some interesting reaction scheme though I lost the source paper on it.
[Edited on 6-12-2020 by mysteriusbhoice]

 Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water.
Urea nitrate may
also be isolated relatively easily from these solutions due to relative low solubility in cold water.
 I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates.
I'm pretty sure to have seen mention of lead/2% silver anodes for nitrate
electrosynthesis somewhere, can't find the reference again... A membrane would prevent cathodic reduction I guess, though indeed, the pH would
decrease probably for the anolyte. Also not sure if such low pH would still allow anodic oxidation to nitrates. 