
vano - 27-4-2021 at 09:17
Hi. There are three compounds, i didn't find stuctures.
•first is Tetraxenonogold(II) distibium undecafluoride. I know cation structure, but how anion looks like?
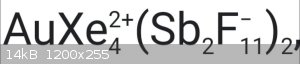
•structure of tetratellurium decaoxotrisulfate. Maybe tellurium has cyclic form, but i don't know structure of whole compound.
•i found interesting tungsten compound. I reduced Phosphotungstic acid with iron sulfate. This brown compound really exists, But I could not see the
structure anywhere.
Copied : Phosphotungstic acid is less sensitive to reduction than phosphomolybdic acid. Reduction with uric acid or iron(II) sulfate produces a brown
coloured compound. the related silicotungstic acid when reduced forms a similar brown compound where one of the four W3 units in the Keggin structure
becomes a metal-metal bonded cluster of three edge shared W(IV) octahedra
Bedlasky - 28-4-2021 at 03:32
Hi.
That brown compound from phosphotungstic acid and iron is interesting.
Tetratellurium trisulfate: Te4S3O10, Te42+ is cyclic cation, look at Woelen's website.
From what I read in this book, it is very hard to prepare Te42+ compound from tellurium and sulfur trioxide, you get mixture of
Te42+ and TeIV. From tellurium and 65% oleum you get TeO(HS2O7)2.
With selenium you can get two pure compounds - from selenium and sulfur trioxide Se4S4O13 and from selenium and 65%
oleum Se4(HS2O7)2.
[Sb2F11]- have structure [F5Sb-F-SbF5]-. It is formed in mixtures of anhydrous HF
and SbF5 along with [SbF6]- (but [Sb2F11]- is predominant species).
[Edited on 28-4-2021 by Bedlasky]
vano - 28-4-2021 at 05:31
Thank you very much Bedlasky. I read about this telluryl(like titanyl or vanadyl?, I ever seen this word) compound. As i remember you gave me this
chalcogens book. Te Trisulfate really exists, but if it looks like for example trichromate, it will be unstable, maybe structure is different.
I thought this structure of fluoroantimony anion, but didn't sure, but now I'm sure Thanks!
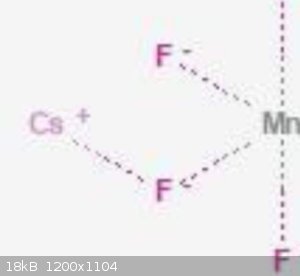
There is Mn, F and Cs compounds, fluorine looks like same.
I can make this brown Mo compound, maybe i will make, we don't have sructure, but it is interesting how stable is this compound. You also can give me
some ideas for experiments for this compound.
Bedlasky - 28-4-2021 at 06:02
Trisulfates exist - they can be made by reaction of pyrosulfate with sulfur trioxide.
vano - 28-4-2021 at 06:47
It Is a logical chemical reaction, but is it stable? I never seen metals salts.
Bedlasky - 28-4-2021 at 09:04
Yes, K2S3O10 is prepared from K2SO4 and SO3. At 150°C it decompose in to K2S2O7 and SO3. Analogous sodium salt also exist. I found this info in Remy
and also in this book. Press ctrl+f and write K2S3O10, you'll find it.
vano - 28-4-2021 at 11:10
Very interesting. thanks!
vano - 30-4-2021 at 06:58
Some of this brown particles really precipitate, but very small amount, i cant filter this.


