
clearly_not_atara - 1-5-2021 at 11:33
The direct fluorination of benzene with CuF2 seems like a solution waiting for a problem:
https://pubs.acs.org/doi/abs/10.1021/op700266y
So I was thinking -- what are the possible uses of fluorobenzene? It has two interesting properties: first, Ar-F undergoes SNAr faster than any other
substituent, and second, it exclusively substitutes at para:
http://doi.org/10.1021/ed080p679
Anyway, this thought was on a shelf for a while. Then I learned that bisphenols with three phenyl linkers are much less estrogenic than bisphenols
with only two phenyl linkers. This is why bisphenol A is hard to replace -- similarly structured bis(4-hydroxyphenyl) compounds are similarly
estrogenic, and alkanols have different reactivity:
https://www.sciencedirect.com/science/article/pii/S088723330...
EDIT: See particularly "BPDB" from:
https://academic.oup.com/toxsci/article/84/2/249/1692264
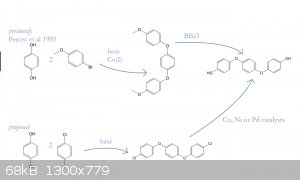
This finally leads to the diagram. The reaction of parachlorofluorobenzene with hydroquinone gives the desired triphenylene structure, and catalytic
Cl->OH substitution gives tri-(p-phenylene)-glycol, or 1,4-bis-(4-hydroxyphenoxy)-benzene.
Previous preparations of this compound relied on boron tribromide to demethylate the dimethyl ether as produced by diarylation of hydroquinone with
4-bromoanisole. This was successfully applied to the synthesis of poly(etheretherether)ketone (PEEEK?):
https://onlinelibrary.wiley.com/doi/pdf/10.1002/pola.1995.08...
I'm not sure about the hydrolysis. There are hundreds of papers on the catalytic hydrolysis of aryl halides, usually with sterically hindered
catalysts to inhibit competing etherification. Some even avoid base altogether. I can't figure out what would be most practical for the substrate,
although there is no shortage of papers that advertise "formation of ethers was not observed".
[Edited on 1-5-2021 by clearly_not_atara]
