
CrudeBasementChemistry - 2-5-2021 at 08:20
hi everyone,
somebody has an idea if titanium is able to withstand 330°C hot nitric acid/ethanol (~50/50) mixture? I guess the nitric acid only should be
reasonable...but in combination with ethanol you'd have both oxidizing and reducing agent together...
mayko - 2-5-2021 at 09:27
"alexa, what's a runaway nitration?"
CrudeBasementChemistry - 2-5-2021 at 12:03
actually it's not a nitration and i would rather get an answer to my question
Fyndium - 2-5-2021 at 12:06
So, making nitroethane in an industrial way?
You should study high temperature reactor material studies and other data. Likely they make that stuff in boro/quartz tube reactors, which is
basically a large tube mounted with long studs and flanges at the end, with possible auxiliary inlet/outlets, heating mantles, etc.
EDIT: Oh, they actually pump BBQ gas with nitric acid to make nitro-alkanes. Well, apparently roasting ethanol with HNO3 produces something
interesting, too.
CrudeBasementChemistry - 3-5-2021 at 00:56
Exactly,
I did so and I know about materials like 1.4361 that can take such harsh reaction conditions, but I still need to purchase tubings or anything like
that made out of it and this is really challenging. Also it should be payable even though I'm willing to spend some money for this project.
Another thing is that is the systems is going to be under a pressure of 3 bar, so glass cannot be used, unfortunately.
I wrote that I wanted to use ethanol/nitric acid mixture, which would only produce nitromethane. But when you switch to 1-propanol you will obtain a
mixture of nitromethane and nitroethane according to this paper (maybe you don't have access, but it's not really important):
https://pubs.acs.org/doi/10.1021/ja01639a028
Of course I want to do both. In this context I'm referring to a patent I found, which states the production of nitromethane from ethanol and nitric
acid with catalystic amounts of dichloroethane:
https://patents.google.com/patent/US4992603
Perhaps it is best to just ask if they also deliver to private persons (for the 1.4361 tubings), but in most cases they do not and if they do you have
to buy a minimum of like 6 meters or something...
And yes, I know that they use propane to make lower nitroalkanes, but 500°C is much higher and corrosion will be a much bigger problem at these
temperatures, also nitropropane would be a side product that is not desired, so this isn't suitable for me.
Fyndium - 3-5-2021 at 01:15
Sci-Hub is an interesting site for accessing research papers.
Generally special alloy steels are available, but with caveat that you have to buy a lot, or find a vendor that supplies smaller amounts. The
industrial suppliers won't even answer to you unless you inquire by MT, or an entire batch which can be 100 tons, depending on the facility size. In
any instance, you will have to pay extra for small purchase. When I bought 4140 steel, I had to buy 6m rod, the supplier was kind enough to cut it
into 1m shafts to allow standard rate shipping. The shipping cost was still equal to material price.
Making the device withstand 3bar is trivial if you tig weld it up. Pressure flanges need only be thick, not round, depending on diameter. Cylindrical
pressure reactors can hence be made with stud rods between two flanges - the tubing itself withstands the pressure with no problem. Biggest issue will
be the gaskets, which may end up pretty costly, or in worst case, you will have to machine hydraulic metal-metal seals, which insists much thicker
flanges to allow for good mating and gas tightness. Not in any way impossible, but you basically must have access to tig, lathe and a mill or a good
drill press.
CrudeBasementChemistry - 3-5-2021 at 01:48
I have access to almost every paper so I hardly need to look for papers on sci-hub, but many others in this forum propably not.
Yes this was my suspicion :/ Anyway I think I will look for a supplier that sells small amounts and ask for it.
Of course 3 bar is not much for SS tubings. My idea is to use SS/Ti tubings for the reactor itself only, otherwise PTFE/PFA tubings that can withstand
up to about 10 bar. Everything with 6 mm outer diameter and connection via swagelok fittings, maybe some parts have to be tig welded, but that is no
problem. I also have access to a lathe and a mill, just in case I need to make some special parts. Since I use swagelok fittings I don't worry about
gas tightness and for those parts that will be connected via flanges I'm going to use grafite gaskets with a metal frame out of 1.4571.
https://www.ebay.de/itm/202245074382?hash=item2f16beedce:g:-...
Maybe I should post a sketch of my setup
Another challenge will be to keep the right temperature. My first thought was a metal bath of molten tin, which would ensure a good heat distribution,
but again I don't know if I had to deal with some kind of corrosion/alloying problems. I'm not aware of any alloys, but you never know...what would be
your opinion on this?
beta4 - 3-5-2021 at 02:40
I remember reading that titanium is incompatible with nitric acid.
Here it is, page 61 of Ignition: https://library.sciencemadness.org/library/books/ignition.pd...
Don't know if it's applicable also to your reaction conditions, but may be a good enough reason to avoid this combination.
CrudeBasementChemistry - 3-5-2021 at 03:04
Poor technician :/ but the question is, as you said if it's applicable to my conditionss. In my case the titanium would only have to deal with 65 %
nitric acid and since it's mixed with ethanol or propanol the concentration is propably under 35 %. Maybe TiO2 isn't even forming due to the reducing
alcohol? Anyway, I will first try to use 1.4571 as it is widely available in Germany and cheap. Also I read somewhere that it is resistant to low
concentrated nitric acid.
By the way, you can find my setup attached.
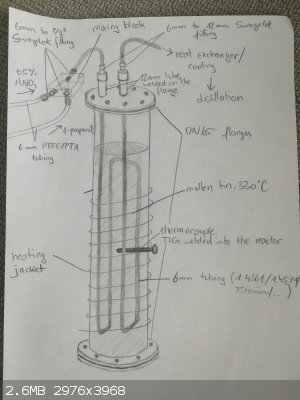
Fyndium - 3-5-2021 at 03:31
That looks fancy. If you proceed with your plan, keep us updated.

