
B(a)P - 26-5-2021 at 04:19
So finally my family is well and truely into our trip around Oz after a few unexpected hurdles. We are doing 9 months around Australia in a camper
trailer. We have reached the part of the journey where we enter the Australian tropics. While we have a freezer, it is only 12 V and making ice does
not seem efficient. So I am wondering would metal cubes be a better option for cooling drinks, maybe copper or silver, or does the energy absorbed by
the melting of ice give a better cooling effect? Or are there other options I am totally missing? Thanks in advance!
draculic acid69 - 26-5-2021 at 05:16
Is the traditional Esky out of the equation or is there a size issue with how much room you have to spare?
Texium - 26-5-2021 at 06:33
Because water has a much higher heat capacity than metals, while it takes more energy to cool down, it inversely requires more energy to heat up. As
you suspected, the heat of fusion of water is also a point in favor of ice. The ice needs to absorb a lot of heat to melt without actually changing
temperature in the process, so you get some “free” cooling power there, as the temperature vs time scale is not linear at the melting point. The
metal cubes may cool off faster, but they’d also warm up very quickly as soon as you place them in your drink, and not contribute much to cooling
it.
If you want to meet in the middle, a similar concept to your metal cubes is whiskey stones. They still don’t work as well as ice in terms of cooling
power, but the stones have a higher heat capacity than metal. https://www.cnn.com/2018/01/18/cnn-underscored/whiskey-stone...
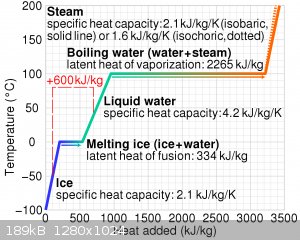
This graph I ripped from Wikipedia shows the cooling power quite nicely. Copper, by comparison has a specific heat capacity of 0.39 kJ/kg/K, so it
would appear practically vertical on that graph compared to ice. Soapstone has a heat capacity of about 1 kJ/kg/K, so still about half that of ice.
And, you can see that most of the ice’s cooling power comes from the act of it melting, which gives it a huge advantage.
B(a)P - 27-5-2021 at 01:20
Thanks very much for that, I was confusing heat capacity with thermal conductivity. This issue with making ice is that on the days we travel, making
ice is not possible because it will all slosh out before it freezes. So maybe it is just cool drinks on the non travel days, I think I can live with
that.
Unfortunately space is at a premium so we can't fit bagged ice or a separate Esky.
Aloesci - 28-5-2021 at 06:41
Aussies where I lived in North Western Australia suprised me by saying that they sometimes put clothes in the freezer.
I also remember putting my wet clothes out on a line and they would be dry in about 5 minutes. I used to carry ice pops everywhere in a cool bag and
eat them constantly.
Gotta love heat capacity.
barbs09 - 29-5-2021 at 03:52
While on the subject of WA, I've heard that to rapidly cool their beers at the end of the day some of the miners would dig a pit, put their beers in,
cover with ammonium nitrate and soak with water. The endothermic cooling on dissolving would make them very cold. Granted its not very practical for
a road trip, and the bags of nitrate you would have to carry around with you might raise the eyebrows of the police if pulled over..
ChemistryGhost - 7-7-2021 at 01:05
You can put computer duster in the freezer or refrigerator. Then wear warm gloves and take it out of the freezer, turn the can upside down, and spray
the side of your drink with it. Do not add it on top of the drink. Computer duster contains difluroethane and a bittering agent. 1,1-Difluoroethane
(DFE) has a boiling point of -13°F (-25°C). You don't have to put the computer duster in the freezer or refrigerator before cooling your drink, but
it maximizes freezing power.
