
ChemistryGhost - 17-1-2022 at 17:35
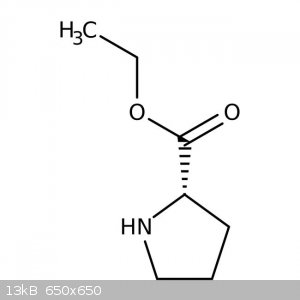
Hello. I was wondering if it's possible to synthesize proline ethyl ester from proline and ethanol using sodium hydroxide, hydrochloric acid, or
sulfuric acid. The goal is freebase proline ethyl ester. Maybe if proline ethyl ester sulfate or proline ethyl ester hydrochloride is made, sodium
hydroxide can be used to freebase it. Then extract with dichloromethane and evaporate the solvent.
Fery - 17-1-2022 at 23:08
It seems you should prepare salt of proline at first. Seems HCl is preferred. Then starting esterification from anhydrous salt of proline. Could be
perhaps done in one pot by mixing proline + anhydrous ethanol and introducing excess of HCl gas so after creating proline hydrochloride the excess of
HCl acts as a catalyst of Fischer esterification. Maybe using Dean-Stark trap + entrainer to remove reaction water would be the best way to go (but
that would force to remove excess of HCl so maybe gassing with HCl would by necessary during whole reaction). To freebase the ester maybe only very
weak base should be used to prevent ester hydrolysis. Maybe NaHCO3 could be enough (I would certainly try as the first attempt) or at most Na2CO3.
Maybe reacting the ester salt dissolved in CH2Cl2 with solid NaHCO3 could work, if not then of course shaking the ester in separatory funnel with
solution of NaHCO3.
https://patents.google.com/patent/CN106083684A/en
In the above patent there is also old route using proline + SOCl2 + methanol in the beginning prior describing their new patent method.
Have you already prepared some easier to synthesize ester(s)?
