
vano - 12-10-2022 at 07:45
Hello! I made cesium hexafluorozirconate. first, I started with zirconium metal. I was surprised because it dissolved very easily in hydrofluoric
acid(conc.). the reaction was fast like the Al&HCl reaction.
I made a reaction in 50ml PP plastic test tube. also, I put this test tube in water, because the reaction was very exothermic. then I added a solution
of cesium sulfate tetrahydrate. nothing happened, I think because the solution was hot. then I put this test tube in the freezer. after 10-15 minutes
I saw a white precipitate. then I filtered it and washed it with distilled water several times.
here is the result:

[Edited on 12-10-2022 by vano]
Boffis - 14-10-2022 at 09:11
Have you analysed the product and what was the yield? The reason I ask is that zirconium is one of the metals to form very stable complexes with
sulphate ions and these complexes mask some of the reaction of zirconium. You should check the stability values for zirconium and fluoride verses
sulphate. You might have a trisulphatozirconate IV complex 
vano - 14-10-2022 at 11:06
thank you! I didn't think that. I know that Zr, Nb, and such metals alkali fluoro complexes are insoluble or not much soluble in water, so I just
precipitated it. ill try to find exactly what it is.
wet powder of the compound must turn the glass opaque after some time, if it is fluoro complex, isn't it?
[Edited on 14-10-2022 by vano]
Boffis - 15-10-2022 at 00:58
Etches glass, Yep that sounds more like a fluoro complex. I don't know how soluble the sulphato-complexes are.
vano - 15-10-2022 at 10:12
one chemist found info for me about sulfatozirconate complexes of alkali metals in an old book. so according to the source, alkali metal
sulfatozirconate will precipitate for example if you add a large exec of sodium chloride in an acidic solution of zirconium sulfate(which contains
exec sulfate anion) Na2[ZrO(SO4)2]*3H2O will precipitate.
also, hydroxytrisulfatezirconate which is in solution loose bisulfate and turns into zyrconyldisulfate anion.
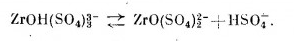
[Edited on 15-10-2022 by vano]

