
indiangold - 29-6-2011 at 00:55
i have done some experiments on epoxidation of d-limonene (4R) and subsequent hydrolysis of the epoxide. the epoxidation (with mcpba or MeCO3H) gives
a mixture of cis and trans epoxides. they are separable by chromatography. when the pure cis or pure trans or the mixture is hydrolyzed with aq.
sulphuric acid, i get only one diol, i.e. the 1s,2s isomer. i had expected the cis isomer to give the 1s,2s,4r isomer and trans to give the 1r,2r,4r
isomer. any advise? or am i making some mistake?
Nicodem - 2-7-2011 at 13:58
I can't imagine a pathway that would give the same diol from both oxirane isomers. Without you giving the full experimental with characterization data
to support your isomer assignations, it is not really possible to give a plausible explanation or point to possible errors.
In principle the "limonene cis-oxide" should give the 1R,2R diol and the "limonene trans-oxide" should give the 1S,2S diol isomer under acidic
conditions. However, due to acidic media further transformations can occur. For example, partial epimerization can occur at the position 1, though the
original diol isomers should still be the major products due to neighbouring group influence. Upon further acid treatment, the diols can rearrange
into both dihydrocarvone epimers. Eventually, further or harsher treatment with acid should cause total loss of optical activity due to various
transformations, like the hydration of the remaining double bond, double bond migrations, aromatizations and oxidations with air. Other reactions are
possible from the diols, like hydration of the double bond, or intramolecular electrophilic addition of the hydroxy groups on the isopropenyl double
bond, but none would lead to a product of the same optical activity from both limonene cis- and trans-oxides.
Note also that there is a difference in reactivity toward acid catalysed ring opening between the two oxiranes: "The greater reactivity of the
cis-epoxide towards acids means that this isomer will give transdihydrocarvone before the trans-epoxide begins to react, and the latter can then be
isolated by fractional distillation." (cited from Natural products review 1989, 291-309)
In fact, I find it surprising that you managed to get any diol instead of the dihydrocarvons directly.
A scheme just to make things comprehensible:
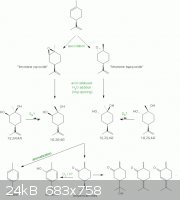
[Edited on 2/7/2011 by Nicodem]
indiangold - 7-7-2011 at 02:01
Dear Nicodem Sir,
I found an article in wikipedia (http://en.wikipedia.org/wiki/Furst-Plattner_Rule). It says something about opening of epoxide with a 2° amine and shows only one regioisomer
isomer being formed, i.e., the amine does not attack the trans epoxide. Only the cis epoxide gives the 2-amino compound. I did not quite understand
this. Any correlation with epoxide opening by water? of course, in my case it is acidic condition, in case of amine it would be basic.
