
Magelia - 11-11-2011 at 11:08
Hi Colleagues,
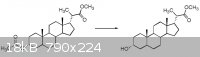
I have this reaction (shown below) I need to perform, but I honestly have NO idea where to start.
The ester at the bottom left is converted into an OH group while the ester at the top right is kept intact.
I honestly have NO clue where to start. Could someone give me a small hint at where to start please? I don't want an answer, just a push in the right
direction... or a direction really at this point 
Thank you!
bahamuth - 11-11-2011 at 11:27
Easiest would probably be to use stoichiometry to saponify just one of the ester groups, release the saponified molecule with acid and separate them
on a good column or on a HPLC.
Just a guess though..
fledarmus - 11-11-2011 at 14:52
You might just try using mass effects to get it to work - stir it in methanol with a catalytic amount of sulfuric acid and possibly a drying agent.
This would set up a transesterification equilibrium between your esters and any available alcohols. The ester on the bottom left would become methyl
acetate and eliminate your sterol alcohol. There is such a large excess of methanol that the chances of your acetic acid forming a new ester with the
sterol alcohol would be negligible.
The ester on the top right would also equilibrate, but since it is already the methyl ester, it will just keep reforming methyl ester with all the
methanol. Again, the methanol is far in excess of the sterol alcohol, so you should get very little of any dimerization.
smuv - 12-11-2011 at 13:35
I agree with fledarmus. At first I thought it was a very difficult problem, but yes, it is as simple as methanol with catalytic acid. A convenient
procedure is to add a catalytic quantity of acetyl chloride to methanol (forms HCl in situ) and then add your ester.
Magelia - 12-11-2011 at 16:42
Thanks a lot guys!
Things really can be very simple sometimes!
ziqquratu - 14-11-2011 at 01:39
It should be pretty easy... I would just stir with K2CO3 in MeOH. The methyl ester that you want to keep intact will remain so, whilst the acetyl
group will be transesterified to give the alcohol and methyl acetate.
Using even a weak base, such as K2CO3, should be faster and more efficient than using acid
Nicodem - 14-11-2011 at 13:22
I agree with ziqquratu that a base catalysed transesterification might be more efficient, but I would point out that the acetate ester function is an
ester of a secondary alcohol and might thus not be so easy to transesterify with K2CO3/MeOH as an ester of a primary alcohol would be. It might
require the use of K3PO4/MeOH or NaOMe/MeOH, which on turn can cause (partial) epimerization at the enolizable chiral carbon. No matter what kind of
methanolysis you use, you would better check that no epimerization occurred. I'm sure it can be avoided though.
smuv - 14-11-2011 at 18:54
I think a literature search is the best bet... This system is pretty common.

