
xwinorb - 30-12-2011 at 16:33
I have two small projects I am looking into right now, and both need a Pd/C reduction of a substituted ketone with different end products.
Case A :
Complete de-oxygenation of the O double bond.
I have one recipe for this case, and I have also exchanged information with two other SM members about it.
In short, the recipe uses a Parr apparatus with a few ATMS of H2, MeOH as the solvent and 5 % Pd/C.
Maybe could be done also w/o the Parr, that's the information I have.
Case B :
Reduction to the alcohol only, not complete de-oxygenation.
I would like if possible to avoid reducing further than the alcohol.
I mean from -C=O to -C(OH). And I would like to COMPLETELY reduce the ketone to the alcohol.
I was thinking about reducing here with with 5 % Pd/C, using ethyl acetate instead of MeOH ( recomended for Case A ), and I would like to know if that
in fact would be a good way to do it or not.
I hope using ethyl acetate instead of MeOH and also the lower pressure will do what I want. Indeed, I am quite inexperienced with the theory and
practice of Pd/C reductions even though I think I can do it.
Also, I would be using a hydrogen ballon instead of a Parr.
Just in case, this projects are still very much in the planning stage, and this is exactly why I am posting. I guess I would not be able to do
anything in less than a few months. I will post any results I get anyway.
Thanks.
xw.
Additional information
xwinorb - 1-1-2012 at 09:48
On case A, the objective is to make 1,4-dimethoxy-2-ethylbenzene from 2,5-dimethoxy-acetophenone. I have already one recipe and more on this one.
On case B, the objective is to reduce :
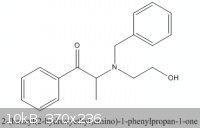
Here I would like to reduce the -C=O to -C-OH,and also to removed the benzyl group connected to the N. It is a protective group.
This partial reduction is achived in this patent by monitoring the H2 absorption, and stopping when it is 2 X the molar amount of the initial
precursor.
Process for the production of substituted Morpholines
US Patent 2,835,669
I would like to do the same withouth having to monitor the amount of H2 absorved, ideally stopping completely at the OH ( not complete deoxygenation
).
I have seen some limited information that ethyl acetate would be more indicated in this case than MeOH, which is a solvent commonly used for Pd/C.
That's all I know.
I have more interest in case B than in case A.
Sorry for the picture quality, I hope to improve it soon.
If someone knows more about it, please post or email me.
xw.
smuv - 3-1-2012 at 12:10
What a long post for such a simple question.
You can probably do both under a balloon.
For the reduction of the acetophenone to the ethylbenzene, you can do it in methanol w. 0.1 - 0.2g/g wet 5% Pd/C (50% water by wt) in methanol
(10-15mL per g) containing 5-10% Conc. hydrochloric acid. Alternatively you may do it in methanol/TFA. You can also do it in straight methanol but
it will probably take longer. I expect the product to be pure after filtering the rxn mixture through celite.
For the second case, to make the phenylpropanolamine deriv. I expect you can do it under a balloon as well. I would say your best bet is MeOH with
2-3 eq HCl or neat acetic acid . Generally benzyl ethers/alcohols are cleaved preferentially to benzylamines, but in this case hydrogenolysis of
phenylethanolamine derivatives is very difficult, I am pretty sure the benzylamine will be cleaved first. You don't have to monitor by hydrogen
absorption, under 1atm of pressure I am nearly positive the hydrogen absorption will essentially stop at your product. If for whatever reason you
observe hydrogenolysis of the ketone you can use Pearlman's catalyst, which is used for reduction of benzylamines in the presence of benzyl alcohols.
[Edited on 1-3-2012 by smuv]
xwinorb - 4-1-2012 at 12:36
To smuv :
Many thanks.
As I said before, it will be sometime till I am able to post any results, but be sure I will as soon as I have something of interest to present. Right
now, work and other projects are keeping me quite busy, it is not lack of interest from my part.
xw.
