
twentypercentcooler - 9-4-2012 at 15:46
I have read about an aldol condensation with benzaldehyde and acetaldehyde, but I find acetaldehyde rather difficult to obtain. I've also heard of
possible routes through cinnamyl alcohol but, I cant seem to find it anywhere. Maybe there are possible routes through styrene? If anyone has any
knowledge on the subject, I would love to know more about cinnamaldehyde synthesis.
Hexavalent - 10-4-2012 at 01:53
Personally, I think this should be in Beginnings. I also assume you want the trans-isomer of the compound.
You can prepare acetaldehyde by slowly dripping acidified dichromate solution onto ethanol in a 2-neck RBF at the BP of acetaldehyde (IIRC ~21*C), and
setting up for distillation on the other neck of the flask with an efficient condenser. This is just a very simple example of primary alcohol
oxidation - it is pretty simple to carry out experimentally, but you may have to be careful and use an excess of alcohol and a controlled amount of
oxidant lest you oxidize it all the way to ethanoic acid. You could also consider going backwards in that regard, using something like Lithium
Aluminium Hydride for carbonyl reduction of the carboxylic acid to the aldehyde - again, you'd have to be careful with your reagents to prevent
excessive reduction.
The following image demonstrates the reductive properties of the LiAlH4, in this case you will need to look at the bottom-right path.

although, considering your limited access to the reagents in your post I am uncertain whether or not you'd have this.
What do you want cinnamaldehyde for anyway? If you specifically want to do the synthesis, for experience, say, then by all means look into doing so.
But, if you just want a sample of the aldehyde for storage or for another experiment, an alternative might be extracting and purifying it from cheap
ground cinnamon powder or even cinnamon bark extract, which IIRC contains often up to 90% cinnamaldehyde. It can also be purchased I believed from
many lab suppliers.
Structure of trans-Cinnamaldehyde, for reference;
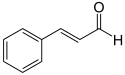
[Edited on 10-4-2012 by Hexavalent]
barley81 - 10-4-2012 at 06:31
Huge thread on acetaldehyde:
http://www.sciencemadness.org/talk/viewthread.php?tid=55
Methods for synthesis include oxidation of ethanol with hydrogen peroxide using vanadium pentoxide as catalyst, oxidation with dichromate, hydration
of acetylene with mercury salts as catalyst, and high-temperature dehydrogenation of ethanol with a copper catalyst.
Benzaldehyde synthesis from toluene is the topic of one of the sticky threads around here.
twentypercentcooler - 10-4-2012 at 12:06
I actually have used dichromate to oxidize ethanol in order to obtain acetaldehyde, but the yeild was very low.
I have heard of reduction of carboxylic acids to their aldehydes, but have not used that method before, I do have some LiAlH4 and I plan to try it
soon.
I already have plenty of benzaldehyde so, that's not a problem.
Also, I was wanting to synthesize some cinnamaldehyde to have some more experience in aldol condensations, and I'm curios about the material itself:
Its metabolites, it's toxicity, its affects on the nervous system via olfactory sense and many other things.
Hexavalent - 10-4-2012 at 12:28
I see, BTW where did you obtain your LiAlH4?
twentypercentcooler - 10-4-2012 at 13:39
I made the LiAlH4 from LiH.
I made the LiH made by direct reaction of molten lithium with hydrogen.
And the lithium was bought from http://www.galliumsource.com/
Number 9 - 12-4-2012 at 04:35
Not sure that the base catalysed aldol synthesis should work. Be aware of the effect of self condensation that occurs when acetaldehyde is added to a
alkaline medium. So, first make a solution of benzaldehyde and a alcoholic NaOH solution is added to it. Add then your acetaldehyde to it with
continue stirring. Neutralize it slowly with HCl solution, until pH 7.
You can isolate it by vacuum destillation, otherwise you destroy your aldehyde by thermal decomposition and fumes are observed. Alternatively, you can
try to isolate it by making a bisulfite addition product, but this product seems fairly stable so, I don't think that this is a good alternative in
this case.
[Edited on 12-4-2012 by Number 9]


