"There is another method of NCl3 producing : from NaOCl , CH3COOH and NH4NO3(any ammonium salt)
The reaction is:
3NaOCl + 2CH3COOH + NH4NO3 = NaNO3 + 2CH3COONa + NCl3 + 3H2O
The reaction doesnt need any control - you just mix the solutions and wait 1-2hours , besides you dont need to work with gaseous chlourine A 5-7% NaOCl solution is used in Russia as a bleach
the method
 make it outdoors because of extremely bad smell!!!)
make it outdoors because of extremely bad smell!!!)reactives :
300ml of 5-7% NaOCl solution ,
50ml of 70% CH3COOH
solution of 10gNH4NO3 in 30ml of water
1)cool the solutions in refredgerator (about -10C)
2) pour 300ml NaOCl into a 0.5l plastic bottle(cola)
3) add 25ml of CH3COOH to NaOCl by small parts
4) add the ammonium nitrate solution to the bottle by small parts(extremely bad smell ! the solution must become yellow)
5) add 25ml CH3COOH to the bottle by small parts
6) leave the bottle(dont close it !) for 1-2 hours The solution must become colourless and a yellow-orange liquid must gather on the bottom (about 10g)
Then close the bottle and throw it somewhere"





 . At high pH it starts bubbling and disappears, I think it forms N2 and hypochlorite
at higher pH.
. At high pH it starts bubbling and disappears, I think it forms N2 and hypochlorite
at higher pH.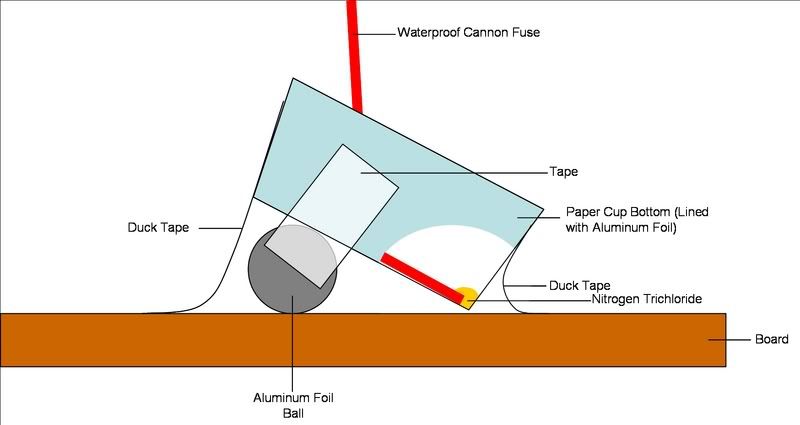



 '
'