endiminion, you should say you had an accident using a flare, flares often contain WP, chances are they will not ask too many questions. say you used
a road flare, or that you were on a boat,...
The flares I have on the boat contain WP, and at one point the company recalled them as they were not safe for use, something about them exploding.
I knew english wasn't your first language, by what I can make out on your P container, it says Fosfor, which means you can live in either:Belgium,
Holland, germany, and a few others.
which one is it?
EDIT:if you are going to say its a welding accident i personally think that you should say that flux dripped onto your hand, even though if you are
welding you should be wearing welding gloves lol.
Just go to ER, even if you tell them you got it purifying WP, what can they do? chances are WP is not illegal, and the cops will have a heck of a time
getting a warrant
[Edited on 1-8-2012 by Pyro] |


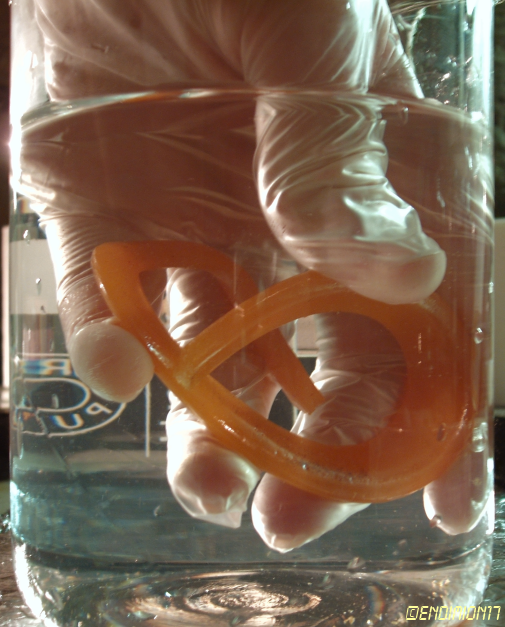
















 was almost burned, and one
piece almost landed on my thigh.
was almost burned, and one
piece almost landed on my thigh.

















 (there are videos on youtube). I had a teacher who used to throw a little peace of
WP in hot, fuming nitric acid - very intense, dangerous reaction - the onset can be slow. If you dissolve a bit of WP in castor oil in a large flask
and shake the flask you can observe very well the fluorescence. A demonstation of the Mitscherlich test is also something interesting to do, but take
care not to inhale the vapours...
(there are videos on youtube). I had a teacher who used to throw a little peace of
WP in hot, fuming nitric acid - very intense, dangerous reaction - the onset can be slow. If you dissolve a bit of WP in castor oil in a large flask
and shake the flask you can observe very well the fluorescence. A demonstation of the Mitscherlich test is also something interesting to do, but take
care not to inhale the vapours...













 )
) 




