Originally posted by maxke
| Code: | 2 H+ + 2e- --> H2 0.00 |
| Code: | 2 Cl- --> Cl2 + 2e- -1.36 |
| Code: | Na --> Na+ + e- 2.71 |
| Code: | 2H2O(l) + 2e- --> H2(g) + 2 OH- -0.83 |
Very interesting effort.
But In the end you only have the four above things going on. I'have been a little out of service in my chemistry exercises ans such but you could
calculate the exact voltage required to stimulate emission of H2/O2 or H2/CL2 given a reservoir of NaCL/H20.
But then there are some energetic losses so it would be best to give a little extra voltage on top. With your 24v I'm sure you are producing CL2.
But ! If you want lots CL2 you need lots electrons. Needing a lot of electrons recquires high current, not a high voltage in my humble opinion.
Just make sure your voltage is high enough so that H2/O2 doesnt form.
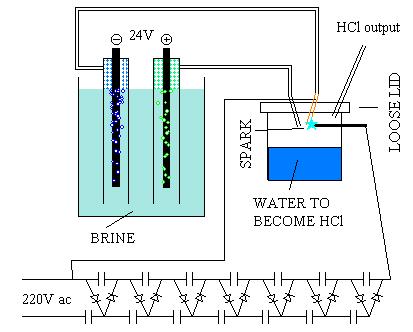
| Code: | Even with a strong brine and 24V (lots of current), not much gas is produced. |
Have you measured the current ?
Preventing CL2 reaction of CL2 with H2O on the electrode position might be stopped introducing a pump, thus leaving the concentration of CL2 on water
level at a minimum.
But then again, it might be better to find a valve or some other device which does the same while doing it explosion INSENSITIVE.
In the end you are still working with explosive gasses.
But my respect goes out to you Tacho.
You have crafted that which I've been thinking about doing it in this or another way.
By the way; where would one acquire electrodes as you have ? |








 sunlight?
sunlight?
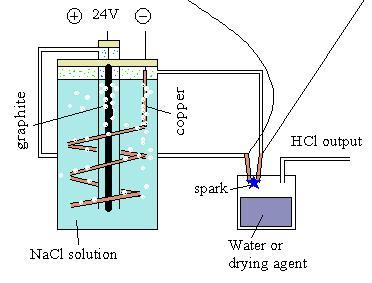


 instead. Other
than that it seems viable.
instead. Other
than that it seems viable.



