
thunderfvck - 24-7-2004 at 01:56
I've been thinking about this for awhile, and I really don't know the answer....Would it be possible to have a sound, at a certain pitch or
tone, that would effectively influence a chemical reaction?
With light, you've got your UV rays and such, and these can provide the energy needed in some reactions. In sound, as far as I know, they're
simply vibrations in the air that eventually reach your ear which you can hear and interpret. Would these vibrations in the air be able to transmit
themselves into a reaction?
Now that I've really started thinking about what sound is, I don't think they influence reactions at all...All sound is is your ear thingee
vibrating at a certain frequency, or whatever...and we interpret sounds from this movement. So how would merely vibrations in air molecules be able to
have an effect on a reaction?
I don't know. I just wanted to hear everyone's opinion. I know there is some kind of field in math/physics or something that deals with
music and how it affects the world or something...I wish I remembered what it was called. I think it started with a "c". Anyways...
I just want to be able to blast some Floyd knowing that it's helping some reaction move along a bit faster...
Geomancer - 24-7-2004 at 07:14
Yes, but ordinary sound doesn't really do much. For real sonochemistry, you need high power ultrasound. The mechanism (for homogenous reactions)
is cavitation providing extremely brief, rapid and intense localized heating. The cavitation bubbles can get hotter than the sun, and under the right
circumstances glow visably (sonoluminescence); I hear that this effect can be created at home. Indeed, there are some reports that even nuclear fusion
can be affected by ultrasound.
Sonochemistry is a popular field, Google should give you more than you want to know.
BromicAcid - 24-7-2004 at 10:08
Yeah, ultrasound is the way to go, a simple ultrasound reaction that I've seen is the production of bromine/hypobromite by the ultrasonic
bombardment of KBr in water.
Since this reaction produces a color change and should not be spontaneous under normal standard state conditions it might be a good test to see if
your ultrasonic production device actually works. Remember sound is a form of energy so it's logical that it can help to induce reactions.
I've made simple ultrasonic devices at home (for undetectably giving people headaches  ) And they're fairly simple to produce, I never ran a test regarding chemistry with it though.
) And they're fairly simple to produce, I never ran a test regarding chemistry with it though.
Cyrus - 25-7-2004 at 21:16
BromicAcid, did they work? I have always wanted the power to give people headaches unnoticed. 
Bring one to work and point it at a neighboring cubicle. That could cause a few reactions.
I'll have to do more research on this, nuclear reactions in the basement sound fun. Not right now though.
I saw an article saying that somniluminescence was useless or impossible (I forget which) in pop. mech., but later they admitted that they might be
wrong.
BromicAcid - 26-7-2004 at 17:18
Don't know if it really worked because if anyone got a headache from it then they never said anything. And I got headaches from it, but that may
have been due to me thinking about it too much.
It did drive dogs crazy almost instantly though, the best results were obtained by fluctuating the frequency between the highest audible and just
beyond, back and fourth. It is my understanding that the most successful devices with this intention utilize this, although I didn't know it at
the time, it is the varying frequency that mostly causes the headache and vertigo.
homemade ultrasonic device?
Vitus_Verdegast - 24-12-2004 at 11:02
How would one utilize a piezo tweeter with 50W, 100W or 150W power to induce sonication enough to promote a chemical reaction? Is it possible to
transfer the energy by just a small layer of air, eg. placing the roundbottom flask directly on the tweeter with a rubber O-ring in between?
Mr. Wizard - 25-12-2004 at 10:44
This may be a little 'off topic', but what are we to think about person who posts with signature that hints at his affection for
cannibalism? Is this a little too weird? I say yes.  I post it here because this
is where I read it last. Maybe some helpful road signs on the road to road to sanity would be in order; such as: this way to the door. Maybe a phone
call to the local constabulary in his area to inquire if any Jeffrey Dalmer wannabes are in the area. Yes freedom of speech is in order, and I can
say what I think. He has already had his.
I post it here because this
is where I read it last. Maybe some helpful road signs on the road to road to sanity would be in order; such as: this way to the door. Maybe a phone
call to the local constabulary in his area to inquire if any Jeffrey Dalmer wannabes are in the area. Yes freedom of speech is in order, and I can
say what I think. He has already had his.
The_Davster - 25-12-2004 at 12:01
I think that the sig came as a result of a poll many months back. I think the poll was called "Human meat, How about it?"
Continue with your thoughts on human flesh in that topic.
Now back on topic...
[Edited on 25-12-2004 by rogue chemist]
HRH_Prince_Charles - 25-12-2004 at 13:28
Mr Verdegast
I doubt that air would provide a strong coupling. You'd need something like a piezoelectric transducer and epoxy resin to get a decent amount of
power into the flask.
Maybe immersing the flask in a powerful ultrasonic bath would do something. I suppose it depends whether you require just ultrasonic mixing or actual
cavitation and such effects.
This is from Feb 1995 Scientific American:
HRH_Prince_Charles - 25-12-2004 at 13:31
I meant to attach this:
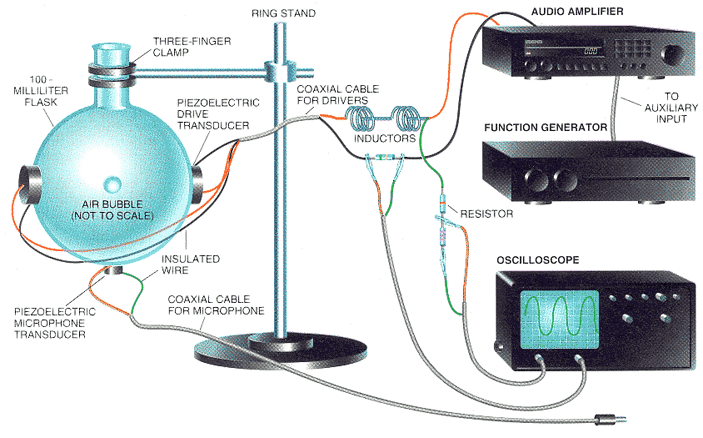
Human Flesh eh?
UpNatom - 25-12-2004 at 14:12
I seem to remember reading something by Magnus Pike (who was an archetypal mad scientist and celebrity in the UK years ago) in which he recounted how
he had proposed to high ranking government officials during the second world war the use of human blood as a foodstuff 
They didn't like that idea at all...but he thought it was perfectly logical
What do you think Vitus?
[sorry for going off topic again...I just thought that was too interesting to leave out]
danke!
Vitus_Verdegast - 26-12-2004 at 09:57
Thank you very much Your Royal Highness!
The eventual purpose is to cause the cavitation necessary for ultrasound-promoted chemical reactions, I doubt a plain ultrasonic bath (like the ones
used to clean jewelry) would suffice here.
Maybe another option would be to construct a probe for immersion in the flask? But how? 

| Quote: |
What do you think Vitus? |
perfectly logical 
HRH_Prince_Charles - 26-12-2004 at 14:53
My pleasure!
I was interested in this a while ago, mainly to use with electrolysis.
Offhand, I can't think of any cheap and easy way to make an ultrasonic probe (sonotrode). They look like expensive items to buy. I wonder whether
a flask immersed in a powerful ultrasound bath would cavitate inside. Maybe the glass would attenuate the sound too much.
http://www.hielscher.com/ultrasonics/sonochem_01.htm
http://www.sonicsandmaterials.com/VCX-500-750/vcx-500-750.ht...
Chas Windsor
Edit: It looks like an ultrasound bath will cavitate the flask contents and can be used for sonochemistry, but a probe gives much more local power. It
might require a high power bath, but I've seen 2nd hand ones go quite cheaply.
[Edited on 26-12-2004 by HRH_Prince_Charles]
Oxydro - 31-12-2004 at 15:33
I'm not too badly off when it comes to ultrasound:


Although I haven't really done much with sonochemistry yet.
mick - 4-1-2005 at 13:23
I have found that if you shout and swear enough the reaction might work
mick

 ) And they're fairly simple to produce, I never ran a test regarding chemistry with it though.
) And they're fairly simple to produce, I never ran a test regarding chemistry with it though.

 I post it here because this
is where I read it last. Maybe some helpful road signs on the road to road to sanity would be in order; such as: this way to the door. Maybe a phone
call to the local constabulary in his area to inquire if any Jeffrey Dalmer wannabes are in the area. Yes freedom of speech is in order, and I can
say what I think. He has already had his.
I post it here because this
is where I read it last. Maybe some helpful road signs on the road to road to sanity would be in order; such as: this way to the door. Maybe a phone
call to the local constabulary in his area to inquire if any Jeffrey Dalmer wannabes are in the area. Yes freedom of speech is in order, and I can
say what I think. He has already had his.






