
Gooferking Science - 21-7-2013 at 19:21
Hello! I am just getting into organic chem, and wanted to know if any of you had some good chlorination experiments to try.
Mercedesbenzene - 21-7-2013 at 21:31
I personally liked the radical chlorination of toluene. It chlorinates the methyl group to yield the mono, di, or tri substituted compound. All of
these are fairly useful compounds. I believe there is something on this subject under the prepublication form. Research the subject yourself to find
out some more information and probably reactions you may be more interested in.
Acyl chlorides
(Brain)2NH - 22-7-2013 at 04:02
converting carboxylic acids to acyl chlorides, then reducing the acyl chloride to yield the mono-chlorinated methyl or even aldehydes.
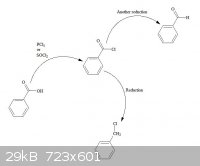
[Edited on 22-7-2013 by (Brain)2NH]
Gooferking Science - 22-7-2013 at 04:09
Thanks for the ideas, and the nice diagram!
Fantasma4500 - 22-7-2013 at 05:47
chlorination of acetic acid
i was looking around for this as for making nitromethane, which can seemingly be done by sodium chloroacetate and sodium nitrite
chloroacetic acid is reacted with sodium hydroxide to get sodium chloroacetate, by what i remember
although its toxic
Gooferking Science - 22-7-2013 at 06:50
Anyone have any tips on the exhaustive halogenation of a methyl ketone in basic conditions? (haloform reaction)
Crowfjord - 22-7-2013 at 08:17
Search the forum for trichloromethane (chloroform) synthesis. It is a fairly simple reaction and has been discussed here many times.
Another beginner friendly reaction would be chlorination of benzyl alcohol to benzyl chloride with excess concentrated hydrochloric acid.
Gooferking Science - 22-7-2013 at 08:24
Where can benzyl alcohol be obtained?
Mailinmypocket - 22-7-2013 at 08:25
Benzyl alcohol...
http://www.ebay.com/bhp/benzyl-alcohol
Gooferking Science - 22-7-2013 at 10:43
Well i mean other than buying off the internet. I only use internet as last resort.
kristofvagyok - 22-7-2013 at 11:41
Chlorination of dioxane is an easily made and a really well going reaction, I can just recommend it!
http://www.sciencemadness.org/talk/viewthread.php?tid=22636
Mercedesbenzene - 22-7-2013 at 16:17
Benzyl alcohol can quite simply be made from an Sn2 reaction of benzyl chloride with some kind of hydroxide. Benzyl chloride can be made from the
chlorination of toluene
Gooferking Science - 22-7-2013 at 16:28
Ah, a chlorination necessary to perform another chlorination.... You know what that means..... CHLORINATIONCEPTION!!!!!!!
Gooferking Science - 22-7-2013 at 16:29
You have to watch the movie inception to understand what I am saying. Great mind boggler of a movie by the way!
