





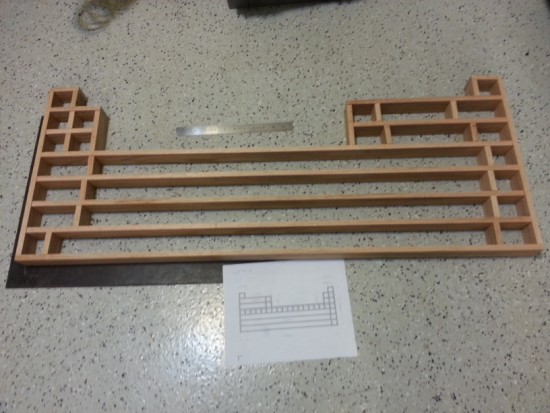
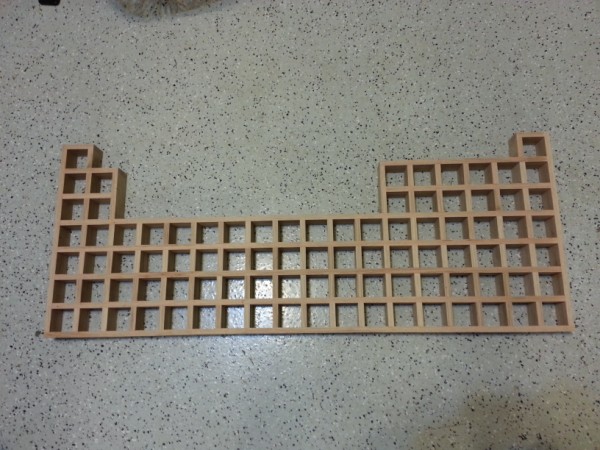
 I finished mine about 6
months ago, after about a year and a half of designing and building. I was lucky enough to have a talented electrical engineer friend that helped me
add fancy LED lighting to mine.
I finished mine about 6
months ago, after about a year and a half of designing and building. I was lucky enough to have a talented electrical engineer friend that helped me
add fancy LED lighting to mine. It really warms the heart to hear that my hobby has influenced
and/or educated other people. Stories like yours really keep me going. I look forward to seeing your finished product!
It really warms the heart to hear that my hobby has influenced
and/or educated other people. Stories like yours really keep me going. I look forward to seeing your finished product!


Quote: Originally posted by MrHomeScientist  |
 ?
?Quote: Originally posted by DubaiAmateurRocketry  |




Quote: Originally posted by bfesser  |
 and two corresponding glass columns. I was
thinking that maybe I could part with one of the plugs, but I'm not sure now (after seeing the price!). I had planned on machining the Teflon into a
more useful item (perhaps a stirrer bearing) when I found a lathe, but it's been years and still no lathe. And now that I have it in front of me, I
realize that it wouldn't even fit in your table without being cut—these things are huge (5 cm height, 7.8 cm max. OD)! If it's alright with
you, I think I'd like to keep them after all, but I'll see if I can scrounge up any other PTFE chunks from my storage totes.
and two corresponding glass columns. I was
thinking that maybe I could part with one of the plugs, but I'm not sure now (after seeing the price!). I had planned on machining the Teflon into a
more useful item (perhaps a stirrer bearing) when I found a lathe, but it's been years and still no lathe. And now that I have it in front of me, I
realize that it wouldn't even fit in your table without being cut—these things are huge (5 cm height, 7.8 cm max. OD)! If it's alright with
you, I think I'd like to keep them after all, but I'll see if I can scrounge up any other PTFE chunks from my storage totes.Quote: Originally posted by bfesser  |

Quote: Originally posted by sargent1015  |
Quote: Originally posted by sonogashira  |






