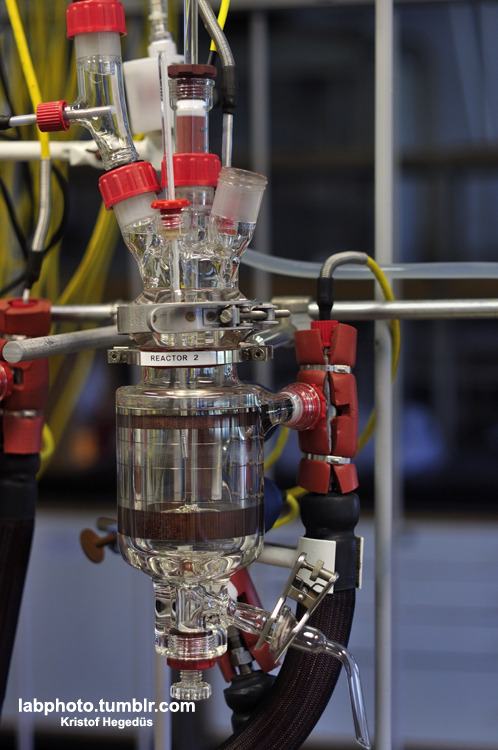


 I'm setting up my first distillation rig,
and asked for the last piece for mmy bday. Should get here on mon. So excited, I'll take a pic. when I get it!
I'm setting up my first distillation rig,
and asked for the last piece for mmy bday. Should get here on mon. So excited, I'll take a pic. when I get it!
Quote: Originally posted by arkoma  |
Quote: Originally posted by Zyklon-A  |


 ), so my lab is looking better than ever. For the first time ever, I got an acid stronger than vinegar
), so my lab is looking better than ever. For the first time ever, I got an acid stronger than vinegar  My parents let me buy some 93% drain cleaner sulfuric acid. Sure, it has impurities, but oh well
My parents let me buy some 93% drain cleaner sulfuric acid. Sure, it has impurities, but oh well  I'll post some pics soon! BTW side note, you can 'purify' sulfuric acid by
distillation, right? My sulfuric acid has dye and corrosion inhibitors in it.
I'll post some pics soon! BTW side note, you can 'purify' sulfuric acid by
distillation, right? My sulfuric acid has dye and corrosion inhibitors in it.



Quote: Originally posted by arkoma  |
Quote: Originally posted by Mailinmypocket  |


Quote: Originally posted by arkoma  |
 Sorry to use this post for anything other than pictures.
Sorry to use this post for anything other than pictures.


Quote: Originally posted by The Volatile Chemist  |
Quote: Originally posted by kristofvagyok  |


| Quote: |
Quote: Originally posted by zts16  |

Quote: Originally posted by arkoma  |
Quote: Originally posted by Loptr  |
Quote: Originally posted by Tsjerk  |

Quote: Originally posted by Mailinmypocket  |

Quote: Originally posted by Mailinmypocket  |
Quote: Originally posted by UnintentionalChaos  |
| Quote: |

Quote: Originally posted by arkoma  |


Quote: Originally posted by arkoma  |

Quote: Originally posted by sasan  |






Quote: Originally posted by Brain&Force  |


Quote: Originally posted by The Volatile Chemist  |
Quote: Originally posted by wish i had a kraken!!!  |
Quote: Originally posted by Zyklon-A  |
Quote: Originally posted by Mailinmypocket  |
Quote: Originally posted by wish i had a kraken!!!  |
Quote: Originally posted by wish i had a kraken!!!  |
Quote: Originally posted by wish i had a kraken!!!  |
Quote: Originally posted by Mailinmypocket  |



Quote: Originally posted by The Volatile Chemist  |
Quote: Originally posted by Mailinmypocket  |






Quote: Originally posted by zts16  |
Quote: Originally posted by zts16  |
Quote: Originally posted by The Volatile Chemist  |