
JAVA - 19-3-2014 at 11:12
I'am currently stucking a bit on the exact mechanism to perform a electrophylic aromatic substitution of acetanilide.
The method that I did do was by adding gently (with cooling) a solution of 5,3 mL bromine in 25mL glacial acetic acid to 13,5g acetanilide dissolved
in 45mL glacial acetic acid. This is exothermic and the main product is however p-bromoacetanilide.
By higher temperatures there is more ortho-bromoacetanilide in the sample, but not that much. (sterical hindrance)
Since I'am writing out the possible side reactions and struggle to write the reaction mechanism of meta-bromoacetanilide I couldn't prove as a
scientist that m-bromoacetanilide exist.
According to my peer-review it's send back to me with the question to write the reaction mechanism of meta-bromoacetanilide. Are there references
(like isotopic labeling, NMR studies) that are helpfull to me in this context?
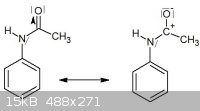
The lone pair on the nitrogen can shift to the carbocation but then this story stops at that point. Maybe the lone pair on the nitrogen stay there but
a nucleophilic attack of acetic acid takes place on the carbocation ?
Short: I don't know it, so I really appreciate some help at this point...
smaerd - 19-3-2014 at 11:58
Hey JAVA,
Edit nevermind I totally misread your question. The wikipedia article on it has a pretty decent explanation of this under the "Ortho/para Directors"
section (en.wikipedia.org/wiki/Electrophilic_aromatic_substitution). Hopefully that gets you moving forward.
[Edited on 19-3-2014 by smaerd]
