

Quote: Originally posted by Brain&Force  |

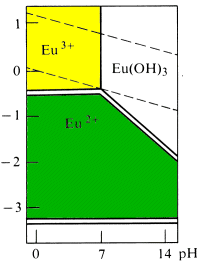
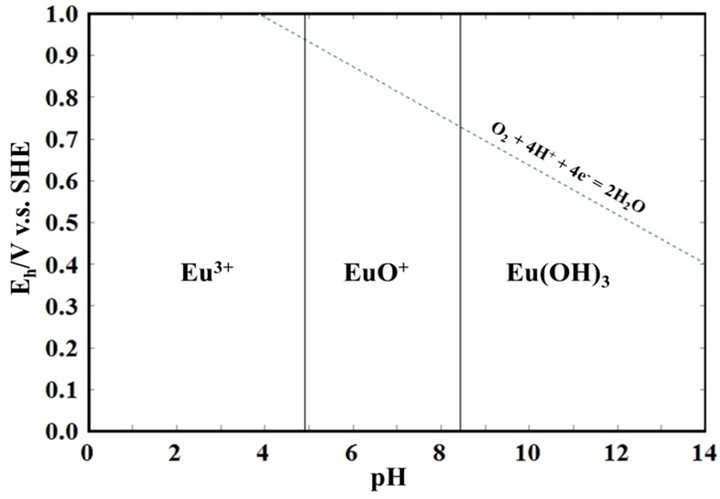
Quote: Originally posted by Brain&Force  |
Quote: Originally posted by Brain&Force  |
Quote: Originally posted by Brain&Force  |
Quote: Originally posted by Brain&Force  |
Quote: Originally posted by MrHomeScientist  |