
Scratch- - 24-2-2005 at 15:38
I have read some of the other posts here on sulfamic acid, I was wondering if I could make H2SO4 in a solution so I dont run the risk of blowing up my
good glassware. When I set up some better apperatus I was going to use the S + KNO3 => SO3 + KN reaction.
neutrino - 24-2-2005 at 17:17
H<sub>2</sub>SO<sub>4</sub> is sulfuric acid
Sulfamic acid = HSO<sub>3</sub>NH<sub>2</sub>
JohnWW - 24-2-2005 at 18:40
In aqueous solution, it would probably exist as a zwitterion, with both negative and positive charges, like an organic amino-acid, (-)SO3-NH3(+).
Scratch- - 25-2-2005 at 12:44
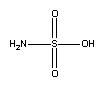
Atually, I looked up its structure (See picture below). Its MSDS says:
"Decomposes with heat (209°C) to release sulfur dioxide (SO2), sulfur trioxide (SO3), nitrogen (N2), water (H2O), and ammonia gas (NH3)."
Also:
"Hazardous reaction in aqueous solution may occur with chlorine, hypochlorous acid, hypochlorites, cyanides, nitric acid, or sulfides. An
explosion occurred when chlorine was being passed at room temperature into a reaction mixture which included sulfamic acid and water. It is suspected
that nitrogen trichloride, a very sensitive explosive, was formed. Fuming nitric acid combined with sulfamic acid causes violent releases of nitrous
oxide."
So wow, I'm glad I know that now.
I was using it to dissolve pennies, which I was trying to get the zinc out of. I was then going to use my pathetic Drano crystals NaOH to precipitate
out fairly pure zinc powder (I would just need to remove the soap and aluminum from the NaOH, which is in clumps). Reverend Necroticus Rex tells me
that zinc sulfamate (Is that name correct?) is a powerful reducing agent.
Do any of you think I could get SO3 (Safely) out of this and thus anhydrous sulfuric acid?
Reverend Necroticus Rex - 25-2-2005 at 19:00
I didn't actually mean that the sulfamate salt itself was a reducing agent, I'm not too sure about that.
I read that in a few MSDS sheets, and I think they might mean, that it erodes the surface layer of oxides on the metal, in a similar way to the action
of HgCl on aluminium foil, as used in for...certain types of organic chemistry

