




 (giving a tingling noise and becoming
conductive) and the diodes simply broke down erratically. Since then I say NO to chinese materials. In the contrary, I have good to very good
experience with old equipment from the former soviet union and the eastern european countries. Many of these things were built ridiculously heavily
and were ridiculously overrated. Not good at all from an economical point of view, but for home experimenting it is very good, some of my old Russian
equipment hardly can be destroyed!
(giving a tingling noise and becoming
conductive) and the diodes simply broke down erratically. Since then I say NO to chinese materials. In the contrary, I have good to very good
experience with old equipment from the former soviet union and the eastern european countries. Many of these things were built ridiculously heavily
and were ridiculously overrated. Not good at all from an economical point of view, but for home experimenting it is very good, some of my old Russian
equipment hardly can be destroyed!Quote: Originally posted by woelen  |
Quote: Originally posted by woelen  |
 I might even order a few of them and sell them on ebay - I can probably post them to the US for about a tenner each.
I might even order a few of them and sell them on ebay - I can probably post them to the US for about a tenner each.




Quote: Originally posted by Lambda-Eyde  |















 I was 2 months old then!
I was 2 months old then!
| Quote: |
Quote: Originally posted by Magpie  |
| Quote: |




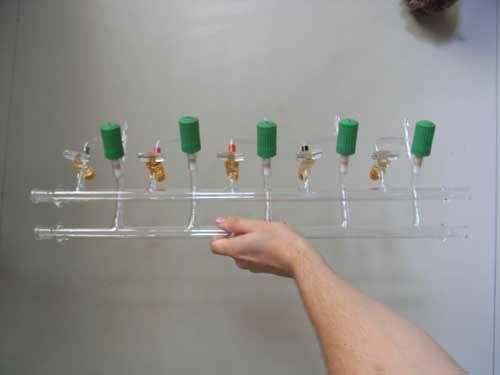
Quote: Originally posted by peach  |
| Quote: |
Quote: Originally posted by pHzero  |

| Quote: |


Quote: Originally posted by pHzero  |
| Quote: |

Quote: Originally posted by The_Davster  |

| Quote: |
Quote: Originally posted by peach  |
Quote: Originally posted by Magpie  |

Quote: Originally posted by entropy51  |
Quote: Originally posted by peach  |
Quote: Originally posted by Magpie  |
Quote: Originally posted by peach  |
Quote: Originally posted by entropy51  |

Quote: Originally posted by psychokinetic  |
Quote: Originally posted by cnidocyte  |











 Way too much equipment, but so little time due to grad school
Way too much equipment, but so little time due to grad school

 .
. .
.Quote: Originally posted by Magpie  |
