
The_Davster - 28-12-2005 at 19:36
So tonight I thought I would have a go making some chloral....
Chlorine source: Ca(OCl)2+10%NaOCl(enough to make semiliquid) + conc H2SO4
Alcohol:15mL 95% EtOH +3 drops of a SbCl3 in HCl solution
So I am dripping the conc sulfuric into the Ca-hypochlorite and bleach slurry a glass tube leading into the ethanol solution. I suddenly see some
suckback and I think...Oh shit I forgot the trap...so to prevent the ethanol solution from getting into the chlorine generator I step up the sulfuric
addition, then suddenly while doing this there is a flash of orange light(in the Cl2 generator) a deep boom...I feel the glass hitting my hunched over
body and I run to the door, halfway there I feel the sting all over my face, I get to the sink inside and rinse the burns with water for 5 min. I
then make a slurry of NaHCO3 and rub it on the burns.
So just what the hell caused this explosion?
See my acid burns in the attached pic. They are like that all over my face, but not as large as the ones by my hairline.




I am going to go rub some more baking soda into the burns...
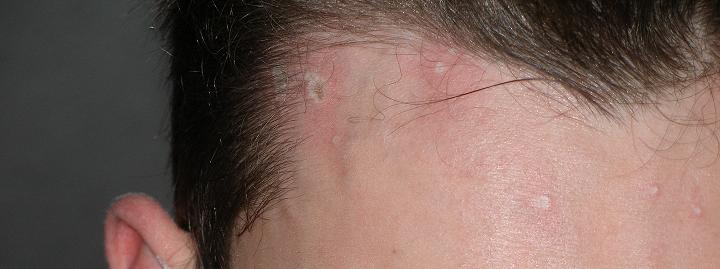
JEEZUS
chloric1 - 28-12-2005 at 19:44
Man you are so lucky! I hope nothing important was damaged(eyes, ears, nose) ! The sucky part about it is when you go out in public people are going
to ask. I hope you have a good story made up. If they find out you where doing home chemistry they could make you the poster child for cracking down
even further on our hobby. Best wishes!
P.S. Many years ago I set alcohol alight merely by adding NaOCl and HCl. I was young and stupid and farting around. The flame burned green because
the chlorine gas was supporting the combustion. Hypochlorites are notoriously
unstable with alcohols. Add acid you have quite a cocktail.
Hypochlorites are notoriously
unstable with alcohols. Add acid you have quite a cocktail.
[Edited on 12/29/2005 by chloric1]
[Edited on 12/29/2005 by chloric1]
Pyrovus - 28-12-2005 at 20:14
I imagine you probably would have got some Cl2O, formed by the dehydrating action of the sulphuric acid on the HOCl formed. I don't know whether it
would have been made in sufficient quantity to be responsible for your explosion, however, given the rapidity at which HOCl decomposes.
chemoleo - 28-12-2005 at 20:19
Wow.
You are lucky it seems. I fared far worse on another occasion. 
Anyway, I am sure the initial irritation will go away fairly soon.
I suppose you have chlorine dioxide in that mix too (nascent), and you can imagine what it does with the ehtanol. Thanks for sharing this, again, it
points out the dangers of suckbacks!
REminds me to an occasion more than a dozen years ago when I tried a small amount of piric acid - and being uncareful as I was, I stained most of my
hands with it. And the bloody stains wouldnt go away (for more than a week!) So at school I said, yah I was helping to paint the house, but wasn't
careful... funny noone believed me ! 
Oh well. All the best. MIght be even good for your hairline, who knows 
BromicAcid - 28-12-2005 at 20:29
Yup, sounds like a concoction for organic hypochlorites, note the thread on the subject. If they accumulated somewhere out of phase (considering they
are hardly water soluble) and were jostled or rubbed in the wrong way that would be a possible side explanation.
Lotek_ - 28-12-2005 at 20:41
from my personal incident, they were actualy kinda cool.
i burned my arm and face kinda bad and after about a day, they didnt hurt at all.
i dunno if it ate the nervs or what but they were much much better than regular burns.
note: my h2so4 was near boiling.
Magpie - 28-12-2005 at 21:35
My sympathies for your unfortunate injury and I hope you heal soon. Tell people that you have had a sudden outbreak of eczema and ask for remedies.
I have never done this reaction but imagine if you were sucking ethanol vapor into a bottle of chlorine there very well could be a rapid oxidation of
the ethanol. This would generate a lot of heat in a confined space with consequent expansion and breakage of the glass?
Lotek_ - 28-12-2005 at 21:48
i just said that i was hurt in chemistry class at school(college). told them that i dropped a beaker of acid... basicly the truth.
The_Davster - 28-12-2005 at 22:48
Thank you all for your concerns and well-wishing
Well the acid burns no longer stick out and are white, they are either red spots, 2 are black spots(one on my hair line, other on ear) and luckily the
majority are just small flesh coloured pits around one or two layers of skin deep. Only about 6 noticeable burns now, I can easily make them out to
be just popped pimples. Still stings though. I do not really need an excuse, I am on christmas break till next semester starts at uni, so till then
I technically do not need to go out into public view other than to go buy some cement for my furnace tomorrow.
Organic hypochlorites, I made ethyl hypochlorite once but I thought those required basic conditions?(And suckback then did not trigger an explosion(I
remember forgetting the trap then too ...))
...))
Well I will inspect the damages to the chlorine generator tomorrow, it is nighttime here, and I must keep out of view of the neighbors, they must have
heard that. I am curious whether the explosion occured in the gas above the slurry, or within the slurry as it could hept determine between ClO2 and
the hypochlorites as the source of the explosion. And there is a syringe of concentrated sulfuric out there which I wish to recover.
EDIT: Chemoleo, what happened with the accident you mentioned?
EDIT2: LOL Chemoleo...I just have a strong widows peak, not a receeding hairline
[Edited on 29-12-2005 by rogue chemist]
Chris The Great - 28-12-2005 at 23:13
Youch! Glad to hear they are clearing up so quickly.
If you act like nothing is wrong people probably won't ask questions.
I am not sure what could have caused the explosion, but I'd guess a suckpack caused the ethanol to burn when it hit the calcium hypochlorite and the
sudden gas pressure caused the explosion.
BromicAcid - 28-12-2005 at 23:19
Actually, probably just the ethanol vapors combusting with the chlorine. The hypochlorite esters are usually formed under basic conditions but they
can take place under acid conditions too, for example, the perchlorate esters can be formed by adding the appropriate alcohol to concentrated
perchloric acid and hope that it doesn't explode, then adding water while hopping that it doesn't explode to precipitate the ester.
chloric1 - 29-12-2005 at 06:24
ethyl hypochlorite? Alcohols and concentrated perchloric acid? (shivers down spine) No thankyou! I would feel safer with nitroglycerine and that is
not saying much. I know tert-butyl hypochlorite is an important reagent in organic chemistry but I would not think primary hypochlorites would be
worth dealing with the danger. But if you like the wild side I guess they could be cool. Could not imagine what an alkyl hypochlorite would smell
like.
Magpie - 29-12-2005 at 09:50
Back to the post mortem:
(Oh, I hope you didn't get any of the fragments/acid in your eyes! You didn't mention any.)
1. A suckback? What caused this? Consumption of Cl2?
2. Is it realistic to find ethanol vapors clear back in your Cl2 generator (We don't know the volumes involved.)
3. The orange flash. That seems like rapid combustion with release of a lot of energy.
4. Just a too rapid generation of Cl2 - this doesn't fit the observations too well.
The_Davster - 29-12-2005 at 13:48
Well I found most of the parts of the gas generator, turns out the glass part survived ...I must have been hit by the metal lid and the tubing. I cannot find one glass tube which was glued through the metal lid, the bubbling
tube or any of the rubber tubing which connected the aparatus. Hopefully it did not end up in the neighbours yard
...I must have been hit by the metal lid and the tubing. I cannot find one glass tube which was glued through the metal lid, the bubbling
tube or any of the rubber tubing which connected the aparatus. Hopefully it did not end up in the neighbours yard , I found the metal lid around 10m from where I was working.
, I found the metal lid around 10m from where I was working.
The generator was still filled with the hypochlorite mixture and as I was washing it out I could smell the unmistakable stench of chlorine dioxide.
So I am leaning towards ethanol combusting in a chlorine dioxide environment.
Marvin - 31-12-2005 at 01:58
I think the answer has been touched on allready, but not underlined. NaOCl and sulphuric acid do not make chlorine at all. Any chlorine produced
must be by contaminating chloride or by decomposition. And as for using concentrated sulphuric acid ;P
Suck back is almost unavoidable, hot oxidiser+sulphuric acid would probably ignite ethanol under most conditions.
The wost accident I had was when I tried to distill about half a litre of methylated spirits with 2 coffie jars, a length of plastic tubing and an
improvised bunson burner. Needless to say the heat made the jar holding the liquid shatter.
Promoting the Healing of Acid Burns
leu - 31-12-2005 at 05:23
Treat your burns with a mixture of natural Vitamin E and a triple antibiotic ointment containing Bactracin, Neomycin and Polymycin B Sulfate 
[Edited on 31-12-2005 by leu]
Thermal - 31-12-2005 at 13:42
I just wanted to add that I had a similar experiance while finishing up a hoffman rearrangement of acetamide with calcium hypochlorite.
Added the NaOH solution to the slurry in a 1l flat bottomed flask, perhaps far too quickly, and "POP" the bloody thing exploded. I made it to the
shower but my eyes were blood red and sore for about 4 days. There were glass shards coming out of my face and hair for a couple days.
it was not the first time I tried the reaction - but it was the first time using that equipment. - Previously I ran the reaction in a beaker and
transfered the slurry to a flask for distillation after.
Never doing chemistry without at least a faceshield again.
[Edited on 31-12-2005 by Thermal]
solo - 31-12-2005 at 15:00
rogue chemist .....Long ago when I used to get paid for providing medical care the ointment with vitamin E was a good rx for burns
tha you suffered leaving no scaring......also any combination of neomycin and retinol will heal you faster due to the vitamin A(retinol) healing
properties.......I thought you might want to know...........solo
BalancedWorld - 12-1-2006 at 01:20
From waccan dairy, alavro sap remove the pain and helps healing, its the pricly plant that grows to about 30cm high. Also good for ingrowing toe
nails.





 Hypochlorites are notoriously
unstable with alcohols. Add acid you have quite a cocktail.
Hypochlorites are notoriously
unstable with alcohols. Add acid you have quite a cocktail.




 ...))
...))
 ...I must have been hit by the metal lid and the tubing. I cannot find one glass tube which was glued through the metal lid, the bubbling
tube or any of the rubber tubing which connected the aparatus. Hopefully it did not end up in the neighbours yard
...I must have been hit by the metal lid and the tubing. I cannot find one glass tube which was glued through the metal lid, the bubbling
tube or any of the rubber tubing which connected the aparatus. Hopefully it did not end up in the neighbours yard , I found the metal lid around 10m from where I was working.
, I found the metal lid around 10m from where I was working.