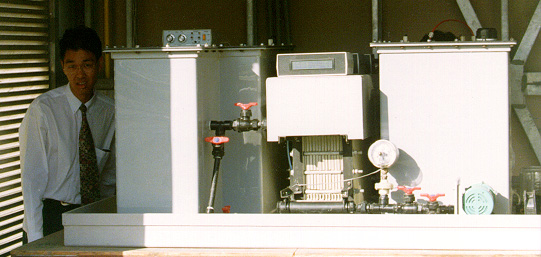
Has anyone here ever done this?
For anyone not familiar, a flow battery is pretty much a reversible fuel cell. Instead of just pumping stuff through it, you have the ability to
charge it regenerating reactants.
Now this is not simply a mental exercise here, I am looking at converting my place to solar, so the idea of a battery that lasts almost forever and
that I could build myself appeals. Depending on my electronics knowledge, hence this thread
I am specifically looking at a vanadium redox battery
http://en.wikipedia.org/wiki/Flow_battery
http://en.wikipedia.org/wiki/Vanadium_redox_battery
http://energystorage.org/energy-storage/technologies/vanadiu...
http://www.vanadium-redox-battery.com/vanadium-redox-battery...
because it seems that regardless of how good your membrane is, you are going to get exchange across so the electrolytes in those cases do not last
forever. So despite vanadium being more expensive than iron and chromium or bromine / polysulfide I want to build this type. Plus, vanadium has nice
colors.
So while I am confident on the chemistry and the physical building, the control electronics are where I am confused.
How do I create a method of charge measurement for the battery? If I overcharge, I will no longer be doing the desired electrochemical reactions, but
instead start on water electrolysis which will change pH and potentially precipitate the V if it continues. So I want some sort of method that when
the cell is fully charged, the charging source is disconnected.
Can any of the electronics gurus recommend anything?
|