
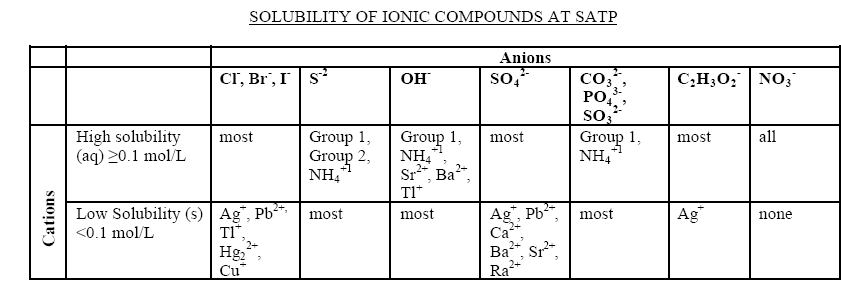
Alain12345 - 30-1-2006 at 10:35
I'm studying for a Grade 11 chemistry exam, and I forgot how to predict precipitate reactions. I know that I have to use the attached chart, but I
forgot how to use it. In my notes, it shows me the steps I have to follow, but can someone explain it to me please?
It says:
Predict possible products
Write dissociation equations
Write full ionic equation (check for solubility of products)
Cancel out spectator ions
Write net ionic equation
Thanks.
12AX7 - 30-1-2006 at 13:46
Ouch, a bitmap. I'm not bothering with that.
I just look for the products that are insoluble. If it's going out of solution, then what's left?
I don't know how thorough you need... it sounds pretty rigorous if you need all those steps, but that's just writing. I mean, you don't even need to
think about KNO3 = K+ + NO3- and such.
Tim
