If you can buy 33/37% HCl but it has impurities in it, simple distillation should remove them and leave you with fairly pure Hydrochloric Acid.
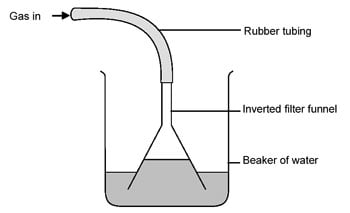
On the other hand, if your store only sells diluted stuff, you may want to concentrate it. HCl and water form an azeotrope that boils at 110ºC and leaves you with 20% concentrated stuff. If you need more concentration, adding Sulfuric Acid and bubbling the resultant HCl gas may be an option...