
warteo - 31-1-2015 at 20:53
Hi, I've got a number of these 250g jars of Ferric ammonium oxalate available for sale -

Great for making cyanotype prints - you'll have to supply your own Potassium ferricyanide though.
Asking $15 each + postage. Really sorry but international shipping is just too much hassle and expense so enquiries from Australian members only
please.
warteo - 11-2-2015 at 18:39
One sold - still have more available.
ahill - 17-2-2015 at 20:39
Hey All - Warteo just dropped one of these off for me - it seems to be the real deal, and so does he.
..just waiting for my potassium ferricyanide now.
Thanks Warteo !
warteo - 17-2-2015 at 23:46
Thanks for the recommendation ahill. Sorry I was in such a rush and had no time for any chemistry chatting but it really was a pleasure to meet you.
Hope you enjoy your "green jelly crystals" 
For anyone else who might be interested, postage to anywhere in Australia works out to $8 for 1 and $12 for 2, 3 or 4 items, paypal or direct deposit.
ahill - 28-2-2015 at 00:27
Got my potassium ferricyanide last week, and today I made my first cyanotype prints.
They worked really well - better than I could have hoped.
This is the first one I ever did :-
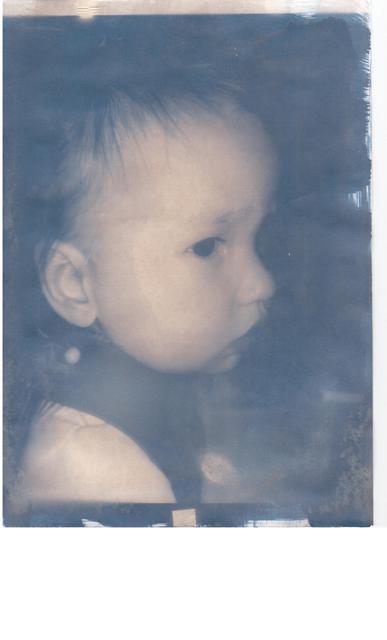
I'm very pleased - I pretty much followed the process here :-
http://www.alternativephotography.com/wp/processes/cyanotype...
..I'd never heard of cyanotype until I saw Warteos post.
On a slightly related note - what do you do with the potassium iron(III) oxalate by-product ? I understand it is kind of toxic.
I made some silver nitrate a few weeks ago - I'd planned to do some silver chloride photography at some point - but this has me itching to get onto it
!
Thanks again Warteo !
Cheers
j_sum1 - 1-3-2015 at 04:15
Nice result ahill. I like the moodiness that is evoked through this kind of image.
I am adding this to the list of projects that I will attempt. I have some potassium ferricyanate coming soon and so with the Ferric ammonium
oxalate, I should be in for some fun.
On a plus side, I think this is a project my wife may be interested in. She has -- shall we say not got the chemistry bug. But a nice pic
of the kids and I think she will be suitably impressed.
On a related note, ahill, what size is the original of the image you posted?
ahill - 1-3-2015 at 15:27
thanks jsum.
the print is on A4 water color paper - the original scan was 2538x4192 - I resized it with Imagemagic's convert.
Its funny, I took what I learned producing the first print, and made 5 duds in a row ! Turns out paper quality and contamination are a real issue. I
was placing the paper on newspaper or kitchen towel when I coated it - when I over-brushed - the brush would get contaminated from the newspaper, and
the whole print would come out crap.
When I worked out what the problem was, I started placing the paper on glass to coat it, and immediately got good results again :-

..so I'd suggest its easy to do - but also easy to screw up.
Cheers
warteo - 3-3-2015 at 18:46
Really fantastic results ahill! Love seeing what you've produced thus far and hearing that you've got the alternative photo process bug happening now

You are correct in your evaluation of paper quality being an issue, residual alkalinity of the paper or the use of hard water are the number one
enemies of this process. These problems can be counteracted to some extent by the appropriate use of dilute HCl or citric/oxalic acids. On a related
note you can use a sodium carbonate solution on the developed print to bleach the image before treating it with tannic acid or various other chemicals
to tone in different colours to the standard blue.
http://unblinkingeye.com/Cyanomicon.pdf Is really worth a look if you want to learn all there is to know about the process.
As for disposal of the potassium iron(III) oxalate by-product crystals, I can't see too much issue with washing down the drain with plenty of water.
There are no toxic heavy metals involved (if you use dichromate, add it to the solution after removing the crystals) and oxalate, while poisonous, is
naturally occurring so the small amounts you'll be disposing of will make no difference.
Will have to do some work to catch up now! I've got quite a few photographic ideas I want to try but all is on the backburner while I spend my spare
time preparing the site for a large shed which will in part be used to house my proper work area. If you listen carefully you can probably hear me
cursing the previous owners and their pile of tonnes of basalt boulders which now need to be removed before I proceed with construction.
j_sum1 - 15-3-2015 at 11:41
Just thought I'd mention that Anna Atkins is featured on Google today -- showcasing her cyanotype prints.




