
Little_Ghost_again - 7-9-2015 at 15:19
Hi All,
Forgive me as its really late and I am very tired, I have been working on something I should have finished ages ago for my school project (Biology), i
thought I had finished it but on giving it the once over I had a doh moment.
I need to measure CO2 about twice a week, I thought simple displacement and upside down volumetric flask, however in my stupidity I totally forgot CO2
readily dissolves in water (dissolves??), and maybe if I had thought about it before I could have come up with some kind of titration etc etc.
But I need quick and simple so I was thinking of displacement of baby oil? or does CO2 dissolve in that as well?
I need to hand in two days times when I next go in, but I dont have much time to mess with it as I am way behind on most of my homework!! (bloody aga
and his challenges!  )
)
blogfast25 - 7-9-2015 at 16:51
L_G_a:
The total CO2 solubility is probably less than you imagine:
https://en.wikipedia.org/wiki/Carbonic_acid#pH_and_compositi...
(see data for p<sub>CO2</sub> = 10<sup>0</sup> atm = 1 atm.
Rather than use messy baby oil (or other organic solvents), use water and apply a correction factor to your results to account for dissolved CO2.
And by positioning the CO2 outlet as in the diagram below you're not bubbling CO2 through the water and dissolution is limited to what little diffuses
into the small, top water surface in the inverted measuring cylinder:
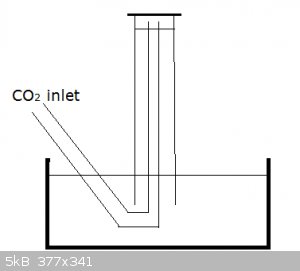
[Edited on 8-9-2015 by blogfast25]
WGTR - 7-9-2015 at 17:39
To give a rough estimate, I've noticed the solubility in water to be about 1:1 by volume at room temperature and atmospheric pressure. The table here gives similar numbers.
Little_Ghost_again - 8-9-2015 at 01:07
Thanks I thought is was higher, I use CO2 in my fish tank and it goes into the main pump hits the impeller and is no more  , I like the tube above the level idea I will do that thanks. Next time I wont leave
it to the last minuet to check
, I like the tube above the level idea I will do that thanks. Next time I wont leave
it to the last minuet to check 

 )
) , I like the tube above the level idea I will do that thanks. Next time I wont leave
it to the last minuet to check
, I like the tube above the level idea I will do that thanks. Next time I wont leave
it to the last minuet to check 