
deltaH - 18-10-2015 at 01:00
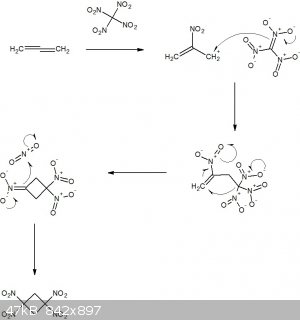
I've drawn up the following hypothetical mechanism for a reaction between propadiene and tetranitromethane and would appreciate some constructive
criticism, please.
Apologies for the 'paper chemistry', I was just having a bit of fun on the breakfast table this morning.
kecskesajt - 18-10-2015 at 03:07
!!!FREEMASON COMPOUND!!!
Seriusly, might work but combining tetranitromethane with reducing agent is not a great idea.
Isn't breaking C=N bond difficult?
j_sum1 - 18-10-2015 at 03:51
Life must be interesting inside your brain deltaH. I don't know where you get these ideas.
That sure is a weird-looking molecule. I wonder what its properties are. I am going to guess reasonably energetic.
http://www.chemspider.com/Chemical-Structure.9854991.html
There is a paper authored by someone named "Flippen-Anderson", which sounds like an attempt to swear politely.
http://scripts.iucr.org/cgi-bin/paper?hh0587
I would be really intrigued if you got this to work.
deltaH - 18-10-2015 at 04:36
p70 of Organic Chemistry of Explosives by Jai Prakash Agrawal, Robert Hodgson has this to say about 1,1,3,3-tetranitrocyclobutane:

********************************************************
Tetranitromethane is extremely toxic, not something I'd fiddle with, but it can be made from the exhaustive nitration of acetylene in the presence of
a little mercury as catalyst. Propadiene is readily available in the form of MAPP gas along with its isomer methyl acetylene and propane, the latter
being inert for wet chemical reactions, so it got me thinking...
I wonder if TNCB could be made by simply bubbling MAPP gas through 100% HNO3 containing mercury nitrate with a good scrubber to capture all the NOx
that would be evolved?
Perhaps just like the acetylene process for making tetranitromethane, this would also require a subsequent treatment with conc. sulfuric acid to drive
the reaction to completion and yield the product.
Probably dangerous wishful thinking, though the if it could be made to work, could be a very low-cost route to an attractive energetic.
[Edited on 18-10-2015 by deltaH]
