
Waffles SS - 15-9-2016 at 20:41
Maleic acid is diacid and polyethylene glycol is diol and i want to make monoester of polyethylene glycol.(this mean one side of glycol is ester)
I make it from maleic anhydride and polyethylene glycol(1:1) but it contain small amount of diester and this act as cross linker in my next step.(my
next step is polymerization of this monomer for making linear polymer)
I am looking for good method for separation mono ester from diester or even making directly monoester without diester .
Some one has experience of this or another mono ester of dicarboxylic acid with Glycols?
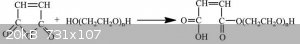
Monoester

Diester
[Edited on 16-9-2016 by Waffles SS]
Metacelsus - 16-9-2016 at 05:32
Could you put a protecting group on one end of your PEG? (If some reacts with the protecting agent at both ends, then you'll have some plain PEG left
over after deprotection. This is probably better for you than having some diester present.)
