
Rhodanide - 11-1-2017 at 08:21
Hi, all.
Got bored and started to make Trimethyl Borate, but got interrupted after I added (more than needed) H2SO4 to some Methanol to dry it. I just
remembered that the reaction of MeOH and conc. H2SO4 makes Dimethyl Sulfate, which is something I do NOT want to mess with...
I'm currently at a different location than my MeOH + H2SO4 mixture (Closed container), and I want to know: should I be worried about potentially
having to handle DMSA when I get back [I want to avoid doing this], can I just proceed with the reaction, and can I dispose of it safely? (That is, if
there was any formed?)
~A-N3
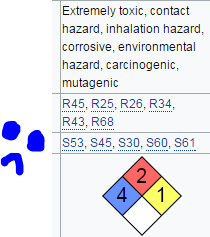
Edit: Descriptive title
[Edited on 1-12-2017 by zts16]
thx
[Edited on 12-1-2017 by Tetra]
violet sin - 11-1-2017 at 18:26
How concentrated was your acid...
Wikipedia- "Me2SO4 has been produced commercially since the 1920s. A common process is the continuous reaction of dimethyl
ether with sulfur trioxide.[7]
(CH3)2O + SO3 → (CH3)2SO4"
----
That doesn't sound like just putting draincleaner in MeOH for a while. But hey, this isn't anything I have read up on either.
Looking around, the mildest condition found listed was 30'C MeOH with CuCl2 catalyst and SO2 bubbled through. Mentioned : http://chemistry.mdma.ch/hiveboard/chemistrydiscourse/000216...
Someone else might be more helpful here, but at least I got some random reading for the day
[Edited on 12-1-2017 by violet sin]
Metacelsus - 11-1-2017 at 20:36
It won't make any appreciable amount of dimethyl sulfate. It might form a little methyl bisulfate, but the equilibrium of MeOH + H2SO4 = MeHSO4 + H2O
greatly favors the left.
Rhodanide - 12-1-2017 at 05:12
Great, thanks guys.
Sin, the concentration was ~96-97/8 %.
Meta, that makes me feel better, haha. The Methyl Bisulfate sounds easier to handle.
