
LD5050 - 17-6-2017 at 22:12
Usually I run very cold mixture of water, ice, and a bunch of salt through my condenser either when I am distilling something or refluxing. I
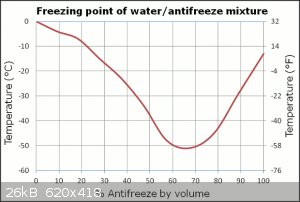
obviously add the salt to the water to lower its freezing point allowing the ice to chill the water bellow 0c. I was thinking tho, what about just
using anti-freeze instead? The concentrated kind and just adding ice to it. The freezing point is much much lower than H2O allowing the ice to cool it
colder then the water, ice, and salt mix.
The reason why I thought of this is because I'm distilling starting fluid and I need the condenser water to be very cold but I ran out of salt and I
had some anti-freeze laying around and I was wondering if it would be a good idea. Anyone have any thoughts?
Tdep - 18-6-2017 at 03:27
Sure, the only issue is adding ice to it will change its freezing point quite a bit, but that's a minor point, especially if you're adding a handful
of ice to a bucketful of EG/water. Rather than diluting it from whatever ratio you've chosen, you have any of those ice pack things? You know, the
freezer brick things that get all cold, you chuck them in you glycol mix and it cools it down without diluting the mix. They're nice, I like using
them.
Sulaiman - 18-6-2017 at 04:05
I would prefer to use something non-toxic such as Glycerol https://en.wikipedia.org/wiki/Glycerol
rather than the more toxic typical Ethylene or Propylene glycol https://en.wikipedia.org/wiki/Antifreeze
unionised - 18-6-2017 at 04:26
It depends refluxing/ distilling.
If it's got a fairly high boiling point- say over 70C- there's not much to be gained by using very cold water (with or w/o anti-freeze) and you
increase the thermal strain on the glassware for no reason.
However if you are trying to get ether to condense then you need something cold.
Glycol / water is rater viscous so you may actually end up with less cooling overall because of the reduced flow rate.
Salt's less of an issue in that regard, but it's more corrosive to some pumps.
phlogiston - 18-6-2017 at 04:28
The viscosity of glycerol could present a problem.
Propylene glycol is not very toxic. It is added to food and is used in e-cigarettes.
PirateDocBrown - 18-6-2017 at 09:07
Dry ice/ Acetone for really cold.
macckone - 18-6-2017 at 09:08
in really low temp mixtures with dry ice and acetone or isopropyl alcohol, the cooling fluid is acetone or isopropyl but you need an explosion proof
motor. You cannot use a cheap plastic submersible
pump.
JJay - 18-6-2017 at 10:00
All submersible pump motors are effectively explosion-proof. The pump itself is required under OSHA regulations to be explosion proof, but they don't
apply to amateurs. I'm not exactly sure what makes a pump explosion-proof, but static discharge is obviously not desirable. The flash point of acetone
is -20 C, so if you're operating under that temperature, I'm not 100% why explosion-proof would be a requirement at all, but obviously you should
follow all local codes and laws no matter how arbitrary or stupid.
That stated, acetone isn't going to play nicely with pump lubricants and sealants unless the pump is designed for pumping solvents, regardless of the
properties of your motor.
BromicAcid - 19-6-2017 at 05:16
Have you ever actually tried to pump dry ice / acetone? Speaking as someone who has tried it, it's darn near impossible. The acetone is saturated
with carbon dioxide, most pump designs generate a low pressure area during their use, this draws liquid into the pump for the discharge stroke, with
the acetone saturated with CO2, it ends up just drawing out CO2 and you cavitate your pump. Now if you're cooling your acetone externally with dry
ice it's a different story but to try to combine the two the way one would while using a cooling bath will likely end in failure.
To the topic of the original post, as unionised said, at higher temperatures it doesn't matter too much. But at lower temperatures it might help you
get that extra edge on cooling. Or if you are running your cooling lines a long distance it will help you get them colder since you're going to warm
up by the time you hit your setup. If you were cooling with something that would otherwise freeze (like an air conditioner compressor) then it would
help you avoid freezing which will help your heat transfer.
Melgar - 19-6-2017 at 08:56
After trying this myself, I found windshield washer fluid works better than all of the above. Reason being it doesn't form a sticky layer over
everything when cleaning up, and you'd be running it cold enough that the boiling point is mostly irrelevant. Also, very cheap and very low freezing
point.
macckone - 26-6-2017 at 19:10
Explosion proof is more of a code requirement.
As long as it is a non-plastic submersible with alcohol (ethanol, isopropanol,
methanol) or acetone resistance. CO2 absorption
in the liquid can be an issue with some pumps.
Dr.Bob - 11-7-2017 at 18:42
We use chillers and glycol mixes to cool our rotovaps, it works fine. That is the ideal way, you could use a small dorm fridge to cool the liquid,
but it won;t keep up for long.
If you use ice, it will eventually dilute it a lot. Better to use sealed cooler bricks or a heat exchanger type system with ice/salt to keep it
cold.
Last choice is dry ice with a dewer type condenser. BTW, I know where you can find those.
curiosity_cat - 14-7-2017 at 16:43
Drill holes for the tubes and just keep your cold solution in the freezer continually chilling , metal container with heat sinks, baseboard heating
pipe with the fins or something, the heat exchangets from old AC unit,
Make it look like science madness.
Do the heat the swimming pool with the AC trick backwards.
[Edited on 15-7-2017 by curiosity_cat]

