
madscientist - 8-7-2002 at 21:36
N10O5 would be an explosive compound of ridiculous power. The structure is two five-membered nitrogen rings (composed of single bonds) joined by five
oxygen atoms. The oxygen atoms are needed, because the two nitrogen rings won't be able to directly join due to the fact that the orbitals that would
bond don't line up. N10O5, to put it in perspective, would liberate approximately 3.4 times as much energy per gram as nitroglycerine.
Now I present all of you with the ultimate challenge: design process for preparation of N10O5. 
Steric Hindrance
PrimoPyro - 8-8-2002 at 18:20
The molecule is too strained to be stable enough to even manufacture.
You want power? Visualize this one:
An all-nitrogen soccer ball comprised of a six membered ring of single bonds, in which alternating nitrogens (1,3,5 for example, and then 2,4 and 6)
attach to another nitrogen "above" and below" the flat (flat in your mind) ring, centered.
The N8 soccerball is one of the most energetic molecules I can think of. Alas, I can't think of a way to make this short of nitrogen laser +
buckyballs, and we all know that isn't practical....
PrimoPyro
madscientist - 8-8-2002 at 18:32
Are you sure? I played with a model of it, and the molecule didn't seem to be as strained as acetone peroxide, for example.
Oops..
PrimoPyro - 8-8-2002 at 18:43
Now that I think again, heh, I was picturing the rings as being FLAT, like aromatic rings, which wouldn't be so for these.
PrimoPyro
kingspaz - 9-8-2002 at 04:30
pyrodine is a benzene ring with an N in place of a CH. the N also takes part in the delocalised system which means the orbitals can overlap (don't
know which ones, edge of my knowledge here as i can;t find any good info about this) so if you had a ring of N's they would only have 2 bonds each and
not be able to bond to other rings.
True
PrimoPyro - 9-8-2002 at 07:21
And that is why I'm sure he was referring to a alkyllic system of only single bonbds. I figured the pyrimidal structure of the nitrogen would be to
strained to be stable.
The nitrogen homolog of benzene isn't even aromatic, and can be reduced with normal methods for reducing azo groups. So, anyone know how to prepare
-N=N-N=N-N=N- (cyclic) ?
Then you can reduce the ring to all imines, and deprotonate and substitute from there.
PrimoPyro
Benzene orbital similar?
Ramiel - 20-8-2002 at 02:48
Would the orbital configuration in Benzene be somewhat similar to the proposed cyclo-pent Nitrogen?
I just thought that because Carbon and Nitrogen share the same bonding orbital in this case right? (the p shell in the third thingie).
If correct, this would mean the electron cloud would be like a halo, above and below the cyclo-nitrogen molecule.
OR!! another confusing revelation... I may well be talking out of my a$$. /: (
Okay, if were all nitrogen storming...
vulture - 20-8-2002 at 08:18
...I have something better!
How about diazideoxide or, even better, diazide peroxide? muhahaha, bet you can't beat that!
Synthesis?
PrimoPyro - 20-8-2002 at 09:35
How do you propose making that? I agree that would be very very energetic, but I don't know how to make such a compound with mild reaction conditions.
And as for maximum energy, wouldn't N8 (with all single bonds, no double bonds like azo groups) be more energetic than any other option, when
comparing equal masses?
PrimoPyro
vulture - 20-8-2002 at 10:08
I have no idea how to prepare my diazides.
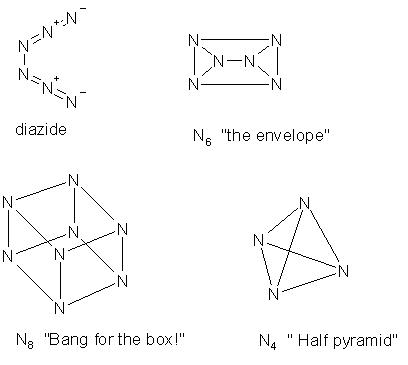
Straight diazide is also possible and what about N6?
Check the imige for some other nitrogens with some trivials I came up with...
cfv - 20-8-2002 at 20:20
How was the figure of 3.4 times NG power found out?
There is an N5+ ion now discovered. See Megalo
mania's forum-high explosives-medina.
HAHA! Ammoniumperoxyazide!
vulture - 21-8-2002 at 08:11
Now this would be an exhilirating compound! NH4-O-O-N3
High nitrogen content and perfect oxygen balance!
Phenylazide might also be an interesting compound to look into and it has been prepared before...
PHILOU Zrealone - 8-9-2002 at 15:30
So you propose (=N-O-N=)5 two pentaaza rings linked via -O- bridges!Funny thing is that I have thought to a similar molecule but C10H15N5
(=CH-NH-CH=)5 that could provide many interesting caged stressed explosive molecules like:
R2N-NO, R2N-NO2, R2NH.HNO3, R2NH.HClO4
so resulting molecules would be:
C10H10N10O5, C10H10N10O10, C10H20N10O15, C10H20N5Cl5O20
All energetic, dense!
Anyway in your case, we would first need to be able to make pentaaza rings (N5X5) with X being H, Cl or F what is not the case!
Octaazacubane is predicted to be very powerful owing to its strained structure...but as always it is only a theorical target molecule since poly N
doesn't like stress at all!
The best of all that might be a little more stable and thus isolable at a not too low temperature and handlable would be hexaazabenzene N6 its perfect
symetry makes it stabler (stil based on theorical calculations).
NH4OON3 won't exist since NH4OONH4 doesn't exist!H2O2 is not a strong enough acid!
N3-O-N3 will be hard to do since NH2-O-NH2 doesn't exist, nor NH2-NH-O-NH-NH2 and because Cl2O exist but the Chlorine atoms aren't negative leaving
groups but positive ones (due to oxygen electronegativity.
The only ways to make it would imply Na2O, Na2O2 and ClN3...thus really unfriendly explosive compounds (often spontaneously) maybe at very low T;
anyway the resulting product can't be handled near ambiant T!
PH Z
N10O5
The_Gender_Changer - 14-11-2004 at 06:01
Madscientist,
could you give me somthing more about
N10O5 and pentaaza-rings .
BromicAcid - 14-11-2004 at 08:33
After discovering the N5+ cation the researchers attempted to react it to produce the species (N5+)(N3-) but they are quoted as saying that their
attempts produced "nothing but explosions" they also said that they think that it cannot exist. Of course that statement has come back to
haunt a number or chemists over the years.
Also, as far as I know at least accurate to 2002, the cyclo N5- anion had only been detected in the gas phase (although compounds contining it are
widely known) it decomposes very quickly though loosing N2 to form N3- but of course they want to find a way to produce it reliably to attempt the
(N5+)(N5-) compound. Although the only route to N5- is via 'electrospray ionization tandem mass spectrometry' at a high collision voltage,
there were recent attempts to produce it though controlled ozonolysis of p-hydroxyphenylpentazole but they failed.
[Much of the information here condensed from Chemical and Engineering News, August 19, 2002, pg. 8, written by Ron Dagani]






