Originally posted by Klute
I think the methylimidazole act exactly like DiMethylAminoPyridine (DMAP) during a Steglich esterification, so it could possibly be substitued by DMAP or equivalent.
I guess other anhydride could be used, butthe formation of the mixed anhydride might be more difficult, needing longer reaction times and possibly
decreasing yields.. but it's just speculation.
Here is my interpretation of the mecanism; I'd appreaciate if someone more knowledgeable on the subject would give his advice on it: ............
|


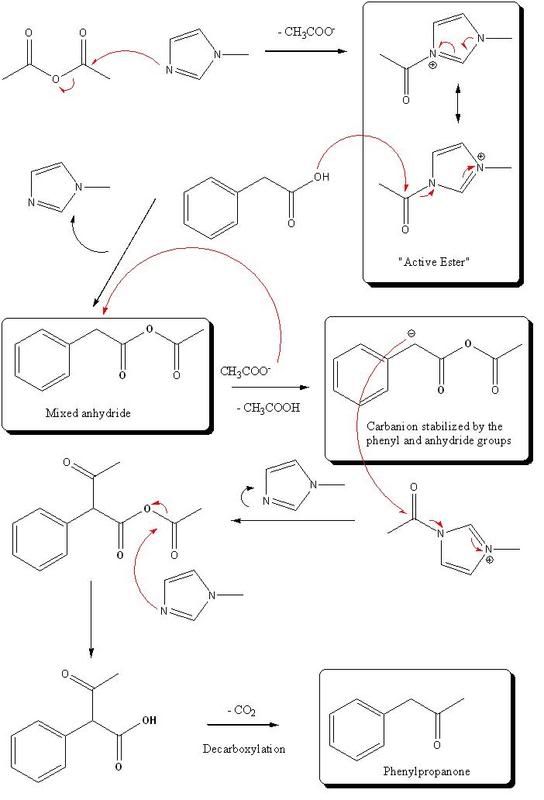



 . Firstly, PAA reacts with acetic anhydride,
creating mixed anhydride; secondly, it is benzylic (α) position attacked and H is "removed"... etc. It is proven , in this and similar
cases, there must be at least two hydrogen atoms attached to this α position. Besides, it is doubtful at all, that acetate anions acts as
"H-abstractors" and ketene mechanism is suggested.
. Firstly, PAA reacts with acetic anhydride,
creating mixed anhydride; secondly, it is benzylic (α) position attacked and H is "removed"... etc. It is proven , in this and similar
cases, there must be at least two hydrogen atoms attached to this α position. Besides, it is doubtful at all, that acetate anions acts as
"H-abstractors" and ketene mechanism is suggested. 

 )
)